Q99JB8
Gene name |
Pacsin3 |
Protein name |
Protein kinase C and casein kinase II substrate protein 3 |
Names |
|
Species |
Mus musculus (Mouse) |
KEGG Pathway |
mmu:80708 |
EC number |
|
Protein Class |
|
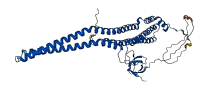
Descriptions
Autoinhibitory domains (AIDs)
Target domain |
16-273 (The F-BAR, FES-CIP4 Homology and Bin/Amphiphysin/Rvs, domain of Protein kinase C and Casein kinase Substrate in Neurons 1, PACSIN1) |
Relief mechanism |
Partner binding |
Assay |
|
Accessory elements
No accessory elements
References
- Yeon JH et al. (2016) "Systems-wide Identification of cis-Regulatory Elements in Proteins", Cell systems, 2, 89-100
- Goh SL et al. (2012) "Versatile membrane deformation potential of activated pacsin", PloS one, 7, e51628
- Shimada A et al. (2010) "Mapping of the basic amino-acid residues responsible for tubulation and cellular protrusion by the EFC/F-BAR domain of pacsin2/Syndapin II", FEBS letters, 584, 1111-8
- Wang Q et al. (2009) "Molecular mechanism of membrane constriction and tubulation mediated by the F-BAR protein Pacsin/Syndapin", Proceedings of the National Academy of Sciences of the United States of America, 106, 12700-5
- Senju Y et al. (2011) "Essential role of PACSIN2/syndapin-II in caveolae membrane sculpting", Journal of cell science, 124, 2032-40
- Rao Y et al. (2010) "Molecular basis for SH3 domain regulation of F-BAR-mediated membrane deformation", Proceedings of the National Academy of Sciences of the United States of America, 107, 8213-8
- Plomann M et al. (2010) "A hinge in the distal end of the PACSIN 2 F-BAR domain may contribute to membrane-curvature sensing", Journal of molecular biology, 400, 129-36
Autoinhibited structure

Activated structure

4 structures for Q99JB8
| Entry ID | Method | Resolution | Chain | Position | Source |
|---|---|---|---|---|---|
| 3M3W | X-ray | 260 A | A/B | 1-320 | PDB |
| 3QE6 | X-ray | 260 A | A/B | 1-304 | PDB |
| 3SYV | X-ray | 310 A | A/B/C/D/E/F/G/H | 1-341 | PDB |
| AF-Q99JB8-F1 | Predicted | AlphaFoldDB |
27 variants for Q99JB8
| Variant ID(s) | Position | Change | Description | Diseaes Association | Provenance |
|---|---|---|---|---|---|
| rs3402727836 | 21 | N>S | No | EVA | |
| rs3388569671 | 26 | V>M | No | EVA | |
| rs3388568574 | 28 | R>K | No | EVA | |
| rs3412845435 | 32 | G>V | No | EVA | |
| rs3391808072 | 78 | E>K | No | EVA | |
| rs3391838162 | 81 | W>C | No | EVA | |
| rs3391890244 | 82 | H>Q | No | EVA | |
| rs3391881622 | 85 | F>S | No | EVA | |
| rs3388571134 | 104 | G>C | No | EVA | |
| rs3388570386 | 116 | G>W | No | EVA | |
| rs3388573337 | 126 | F>Y | No | EVA | |
| rs3388570364 | 133 | E>D | No | EVA | |
| rs3388570382 | 137 | R>H | No | EVA | |
| rs3388566490 | 152 | A>P | No | EVA | |
| rs3388569642 | 170 | R>P | No | EVA | |
| rs3388568629 | 171 | E>* | No | EVA | |
| rs3388570315 | 172 | S>T | No | EVA | |
| rs3388569636 | 196 | T>I | No | EVA | |
| rs3388565005 | 222 | E>* | No | EVA | |
| rs3388564737 | 227 | A>D | No | EVA | |
| rs3388564765 | 231 | C>Y | No | EVA | |
| rs3388563178 | 256 | S>F | No | EVA | |
| rs241555783 | 271 | S>G | No | EVA | |
| rs3388570053 | 313 | K>E | No | EVA | |
| rs3388561102 | 343 | S>T | No | EVA | |
| rs3388572429 | 344 | S>F | No | EVA | |
| rs3391865334 | 361 | K>T | No | EVA |
No associated diseases with Q99JB8
Functions
4 GO annotations of cellular component
| Name | Definition |
|---|---|
| cytoplasm | The contents of a cell excluding the plasma membrane and nucleus, but including other subcellular structures. |
| cytosol | The part of the cytoplasm that does not contain organelles but which does contain other particulate matter, such as protein complexes. |
| endosome | A vacuole to which materials ingested by endocytosis are delivered. |
| plasma membrane | The membrane surrounding a cell that separates the cell from its external environment. It consists of a phospholipid bilayer and associated proteins. |
5 GO annotations of molecular function
| Name | Definition |
|---|---|
| calcium channel inhibitor activity | Binds to and stops, prevents, or reduces the activity of a calcium channel. |
| cytoskeletal protein binding | Binding to a protein component of a cytoskeleton (actin, microtubule, or intermediate filament cytoskeleton). |
| identical protein binding | Binding to an identical protein or proteins. |
| lipid binding | Binding to a lipid. |
| phospholipid binding | Binding to a phospholipid, a class of lipids containing phosphoric acid as a mono- or diester. |
7 GO annotations of biological process
| Name | Definition |
|---|---|
| cytoskeleton organization | A process that is carried out at the cellular level which results in the assembly, arrangement of constituent parts, or disassembly of cytoskeletal structures. |
| endocytosis | A vesicle-mediated transport process in which cells take up external materials or membrane constituents by the invagination of a part of the plasma membrane to form a new membrane-bounded vesicle. |
| negative regulation of calcium ion transport | Any process that stops, prevents, or reduces the frequency, rate or extent of the directed movement of calcium ions into, out of or within a cell, or between cells, by means of some agent such as a transporter or pore. |
| negative regulation of endocytosis | Any process that stops, prevents, or reduces the frequency, rate or extent of endocytosis. |
| plasma membrane tubulation | A membrane tubulation process occurring in a plasma membrane. |
| positive regulation of membrane protein ectodomain proteolysis | Any process that activates or increases the frequency, rate or extent of membrane protein ectodomain peptidolysis. |
| regulation of endocytosis | Any process that modulates the frequency, rate or extent of endocytosis. |
15 homologous proteins in AiPD
| UniProt AC | Gene Name | Protein Name | Species | Evidence Code |
|---|---|---|---|---|
| P25623 | SYP1 | Suppressor of yeast profilin deletion | Saccharomyces cerevisiae (strain ATCC 204508 / S288c) (Baker's yeast) | PR |
| A7MBI0 | PACSIN1 | Protein kinase C and casein kinase substrate in neurons protein 1 | Bos taurus (Bovine) | SS |
| O13154 | PACSIN2 | Protein kinase C and casein kinase substrate in neurons protein 2 | Gallus gallus (Chicken) | SS |
| Q9BY11 | PACSIN1 | Protein kinase C and casein kinase substrate in neurons protein 1 | Homo sapiens (Human) | EV |
| Q9UNF0 | PACSIN2 | Protein kinase C and casein kinase substrate in neurons protein 2 | Homo sapiens (Human) | EV |
| Q9UKS6 | PACSIN3 | Protein kinase C and casein kinase substrate in neurons protein 3 | Homo sapiens (Human) | SS |
| Q9H939 | PSTPIP2 | Proline-serine-threonine phosphatase-interacting protein 2 | Homo sapiens (Human) | PR |
| Q61644 | Pacsin1 | Protein kinase C and casein kinase substrate in neurons protein 1 | Mus musculus (Mouse) | EV |
| Q9WVE8 | Pacsin2 | Protein kinase C and casein kinase substrate in neurons protein 2 | Mus musculus (Mouse) | EV |
| P97814 | Pstpip1 | Proline-serine-threonine phosphatase-interacting protein 1 | Mus musculus (Mouse) | PR |
| Q99M15 | Pstpip2 | Proline-serine-threonine phosphatase-interacting protein 2 | Mus musculus (Mouse) | PR |
| Q60780 | Gas7 | Growth arrest-specific protein 7 | Mus musculus (Mouse) | PR |
| Q9Z0W5 | Pacsin1 | Protein kinase C and casein kinase substrate in neurons protein 1 | Rattus norvegicus (Rat) | SS |
| Q9QY17 | Pacsin2 | Protein kinase C and casein kinase substrate in neurons 2 protein | Rattus norvegicus (Rat) | SS |
| Q4V920 | pacsin1b | Protein kinase C and casein kinase substrate in neurons protein 1 | Danio rerio (Zebrafish) (Brachydanio rerio) | SS |
| 10 | 20 | 30 | 40 | 50 | 60 |
| MAPEEDAGGE | VLGGSFWEAG | NYRRTVQRVE | DGHRLCGDLV | SCFQERARIE | KAYAQQLADW |
| 70 | 80 | 90 | 100 | 110 | 120 |
| ARKWRGAVEK | GPQYGTLEKA | WHAFFTAAER | LSELHLEVRE | KLHGPDSERV | RTWQRGAFHR |
| 130 | 140 | 150 | 160 | 170 | 180 |
| PVLGGFRESR | AAEDGFRKAQ | KPWLKRLKEV | EASKKSYHTA | RKDEKTAQTR | ESHAKADSSM |
| 190 | 200 | 210 | 220 | 230 | 240 |
| SQEQLRKLQE | RVGRCTKEAE | KMKTQYEQTL | AELNRYTPRY | MEDMEQAFES | CQAAERQRLL |
| 250 | 260 | 270 | 280 | 290 | 300 |
| FFKDVLLTLH | QHLDLSSSDK | FHELHRDLQQ | SIEAASDEED | LRWWRSTHGP | GMAMNWPQFE |
| 310 | 320 | 330 | 340 | 350 | 360 |
| EWSLDTQRAI | SRKEKGGRSP | DEVTLTSIVP | TRDGTAPPPQ | SPSSPGSGQD | EDWSDEESPR |
| 370 | 380 | 390 | 400 | 410 | 420 |
| KVATGVRVRA | LYDYAGQEAD | ELSFRAGEEL | LKMSEEDEQG | WCQGQLQSGR | IGLYPANYVE |
| CVGA |