Q8Y842
Gene name |
lmo1076 |
Protein name |
Lmo1076 protein |
Names |
|
Species |
Listeria monocytogenes serovar 1/2a (strain ATCC BAA-679 / EGD-e) |
KEGG Pathway |
lmo:lmo1076 |
EC number |
|
Protein Class |
PEPTIDOGLYCAN HYDROLASE FLGJ (PTHR33308) |
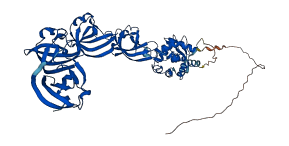
Descriptions
Auto (lmo1076) is one of autolysins and is essential for listerial entry into numerous cell lines. The α helix in the N-terminal region (60-72) of Auto physically blocks the substrate-binding cleft of the protein.
Autoinhibitory domains (AIDs)
Target domain |
89-243 (Peptidoglycan hydrolase domain) |
Relief mechanism |
Cleavage |
Assay |
Deletion assay, Structural analysis |
Accessory elements
No accessory elements
Autoinhibited structure
Activated structure

3 structures for Q8Y842
| Entry ID | Method | Resolution | Chain | Position | Source |
|---|---|---|---|---|---|
| 3FI7 | X-ray | 235 A | A | 52-243 | PDB |
| 5JQC | X-ray | 215 A | A | 233-491 | PDB |
| AF-Q8Y842-F1 | Predicted | AlphaFoldDB |
No variants for Q8Y842
| Variant ID(s) | Position | Change | Description | Diseaes Association | Provenance |
|---|---|---|---|---|---|
| No variants for Q8Y842 | |||||
No associated diseases with Q8Y842
Functions
| Description | ||
|---|---|---|
| EC Number | ||
| Subcellular Localization |
|
|
| PANTHER Family | PTHR33308 | PEPTIDOGLYCAN HYDROLASE FLGJ |
| PANTHER Subfamily | PTHR33308:SF9 | PEPTIDOGLYCAN HYDROLASE FLGJ |
| PANTHER Protein Class |
metabolite interconversion enzyme
hydrolase |
|
| PANTHER Pathway Category | No pathway information available | |
1 GO annotations of cellular component
| Name | Definition |
|---|---|
| extracellular region | The space external to the outermost structure of a cell. For cells without external protective or external encapsulating structures this refers to space outside of the plasma membrane. This term covers the host cell environment outside an intracellular parasite. |
1 GO annotations of molecular function
| Name | Definition |
|---|---|
| amidase activity | Catalysis of the reaction: a monocarboxylic acid amide + H2O = a monocarboxylate + NH3. |
No GO annotations of biological process
| Name | Definition |
|---|---|
| No GO annotations for biological process |
No homologous proteins in AiPD
| UniProt AC | Gene Name | Protein Name | Species | Evidence Code |
|---|---|---|---|---|
| No homologous proteins | ||||
| 10 | 20 | 30 | 40 | 50 | 60 |
| MIKKVFHFIL | VLMLSVSIIP | LFHAKAAETT | NGVDSSEQED | NTEVAREEMP | PESEEPVFSL |
| 70 | 80 | 90 | 100 | 110 | 120 |
| EQNRDDAMAA | LVVPQTRNSF | LRAASTPTFQ | QTFINSISTQ | AMDLCKKYNL | YPSVMIAQAA |
| 130 | 140 | 150 | 160 | 170 | 180 |
| LESNWGRSEL | GKAPNYNLFG | IKGSYNGKSV | TMKTWEYSDS | KGWYQINANF | AKYPSHKESL |
| 190 | 200 | 210 | 220 | 230 | 240 |
| EDNAKKLRNG | PSWDSSYYKG | AWRENAKTYK | DATAWLQGRY | ATDNTYASKL | NTLISSYNLT |
| 250 | 260 | 270 | 280 | 290 | 300 |
| QYDTLYDTIK | QQKNVSEDAK | VVKADGHGVY | SGIYNTSAAS | AKKLSTGAPY | NNKDVKILKE |
| 310 | 320 | 330 | 340 | 350 | 360 |
| GTTSRGTWVQ | FSLNNKVIGW | MDKRAFVYYP | KATNVKTLNL | TGKITAGSTN | GLWSEVPGTV |
| 370 | 380 | 390 | 400 | 410 | 420 |
| NAKKLATTAG | AYQNKDAKII | KQGQISGRTY | YQFQVGGKTI | GWLDARAFHV | YDKIQSQSNV |
| 430 | 440 | 450 | 460 | 470 | 480 |
| NWNRTILNAD | KHGVYSGVYN | TSSSSMNKLS | TGAKYNNKKV | KVIKQAKTAR | GTWYQFQVNG |
| 490 | 500 | 510 | 520 | 530 | 540 |
| KTVGWMDYRA | FFDTITSQKT | MNKTVTVGNA | TNHGVFDGVY | RTSPTVKRIS | LGKPYNNKKV |
| 550 | 560 | 570 | |||
| KVLKEAVTDH | ATWVQFKYGN | TTAWMDKKAF | KY |