Q8VHF0
Gene name |
Mark3 |
Protein name |
MAP/microtubule affinity-regulating kinase 3 |
Names |
EC 2.7.11.1 |
Species |
Rattus norvegicus (Rat) |
KEGG Pathway |
rno:170577 |
EC number |
2.7.11.1: Protein-serine/threonine kinases |
Protein Class |
|
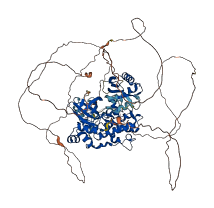
Descriptions
Autoinhibitory domains (AIDs)
Target domain |
56-307 (Protein kinase domain) |
Relief mechanism |
Others |
Assay |
|
Accessory elements
195-217 (Activation loop from InterPro)
Target domain |
56-307 (Protein kinase domain) |
Relief mechanism |
|
Assay |
|
Autoinhibited structure

Activated structure

1 structures for Q8VHF0
| Entry ID | Method | Resolution | Chain | Position | Source |
|---|---|---|---|---|---|
| AF-Q8VHF0-F1 | Predicted | AlphaFoldDB |
No variants for Q8VHF0
| Variant ID(s) | Position | Change | Description | Diseaes Association | Provenance |
|---|---|---|---|---|---|
| No variants for Q8VHF0 | |||||
No associated diseases with Q8VHF0
6 regional properties for Q8VHF0
| Type | Name | Position | InterPro Accession |
|---|---|---|---|
| domain | Protein kinase domain | 56 - 307 | IPR000719 |
| domain | Kinase associated domain 1 (KA1) | 748 - 797 | IPR001772 |
| active_site | Serine/threonine-protein kinase, active site | 174 - 186 | IPR008271 |
| domain | Ubiquitin-associated domain | 326 - 365 | IPR015940 |
| binding_site | Protein kinase, ATP binding site | 62 - 85 | IPR017441 |
| domain | Serine/threonine-protein kinase MARK 1-4, catalytic domain | 55 - 307 | IPR049508 |
Functions
| Description | ||
|---|---|---|
| EC Number | 2.7.11.1 | Protein-serine/threonine kinases |
| Subcellular Localization |
|
|
| PANTHER Family | ||
| PANTHER Subfamily | ||
| PANTHER Protein Class | ||
| PANTHER Pathway Category | No pathway information available | |
3 GO annotations of cellular component
| Name | Definition |
|---|---|
| cytoplasm | The contents of a cell excluding the plasma membrane and nucleus, but including other subcellular structures. |
| dendrite | A neuron projection that has a short, tapering, morphology. Dendrites receive and integrate signals from other neurons or from sensory stimuli, and conduct nerve impulses towards the axon or the cell body. In most neurons, the impulse is conveyed from dendrites to axon via the cell body, but in some types of unipolar neuron, the impulse does not travel via the cell body. |
| plasma membrane | The membrane surrounding a cell that separates the cell from its external environment. It consists of a phospholipid bilayer and associated proteins. |
4 GO annotations of molecular function
| Name | Definition |
|---|---|
| ATP binding | Binding to ATP, adenosine 5'-triphosphate, a universally important coenzyme and enzyme regulator. |
| protein serine kinase activity | Catalysis of the reactions |
| protein serine/threonine kinase activity | Catalysis of the reactions |
| tau-protein kinase activity | Catalysis of the reaction |
8 GO annotations of biological process
| Name | Definition |
|---|---|
| intracellular signal transduction | The process in which a signal is passed on to downstream components within the cell, which become activated themselves to further propagate the signal and finally trigger a change in the function or state of the cell. |
| microtubule cytoskeleton organization | A process that is carried out at the cellular level which results in the assembly, arrangement of constituent parts, or disassembly of cytoskeletal structures comprising microtubules and their associated proteins. |
| negative regulation of hippo signaling | Any process that stops, prevents, or reduces the frequency, rate or extent of hippo signaling. |
| negative regulation of protein localization to nucleus | Any process that stops, prevents or reduces the frequency, rate or extent of protein localization to nucleus. |
| peptidyl-serine autophosphorylation | The phosphorylation by a protein of one or more of its own serine amino acid residues, or a serine residue on an identical protein. |
| peptidyl-serine phosphorylation | The phosphorylation of peptidyl-serine to form peptidyl-O-phospho-L-serine. |
| positive regulation of protein binding | Any process that activates or increases the frequency, rate or extent of protein binding. |
| protein phosphorylation | The process of introducing a phosphate group on to a protein. |
18 homologous proteins in AiPD
| UniProt AC | Gene Name | Protein Name | Species | Evidence Code |
|---|---|---|---|---|
| Q96L34 | MARK4 | MAP/microtubule affinity-regulating kinase 4 | Homo sapiens (Human) | SS |
| P57059 | SIK1 | Serine/threonine-protein kinase SIK1 | Homo sapiens (Human) | PR |
| Q7KZI7 | MARK2 | Serine/threonine-protein kinase MARK2 | Homo sapiens (Human) | SS |
| Q9P0L2 | MARK1 | Serine/threonine-protein kinase MARK1 | Homo sapiens (Human) | EV |
| P27448 | MARK3 | MAP/microtubule affinity-regulating kinase 3 | Homo sapiens (Human) | SS |
| Q8VHJ5 | Mark1 | Serine/threonine-protein kinase MARK1 | Mus musculus (Mouse) | SS |
| Q8C0N0 | Gm4922 | Sperm motility kinase Z | Mus musculus (Mouse) | PR |
| Q8C0X8 | Sperm motility kinase X | Mus musculus (Mouse) | PR | |
| Q8CIP4 | Mark4 | MAP/microtubule affinity-regulating kinase 4 | Mus musculus (Mouse) | SS |
| A0AUV4 | Gm7168 | Sperm motility kinase Y | Mus musculus (Mouse) | PR |
| Q05512 | Mark2 | Serine/threonine-protein kinase MARK2 | Mus musculus (Mouse) | SS |
| Q03141 | Mark3 | MAP/microtubule affinity-regulating kinase 3 | Mus musculus (Mouse) | SS |
| O08678 | Mark1 | Serine/threonine-protein kinase MARK1 | Rattus norvegicus (Rat) | SS |
| O08679 | Mark2 | Serine/threonine-protein kinase MARK2 | Rattus norvegicus (Rat) | SS |
| Q852Q1 | OSK4 | Serine/threonine protein kinase OSK4 | Oryza sativa subsp. japonica (Rice) | SS |
| Q852Q2 | OSK1 | Serine/threonine protein kinase OSK1 | Oryza sativa subsp. japonica (Rice) | SS |
| Q852Q0 | OSK3 | Serine/threonine protein kinase OSK3 | Oryza sativa subsp. japonica (Rice) | SS |
| Q9TW45 | par-1 | Serine/threonine-protein kinase par-1 | Caenorhabditis elegans | SS |
| 10 | 20 | 30 | 40 | 50 | 60 |
| MSTRTPLPTV | NERDTENHIS | HGDGRQEVTS | RTGRSGARCR | NSIASCADEQ | PHIGNYRLLK |
| 70 | 80 | 90 | 100 | 110 | 120 |
| TIGKGNFAKV | KLARHILTGR | EVAIKIIDKT | QLNPTSLQKL | FREVRIMKIL | NHPNIVKLFE |
| 130 | 140 | 150 | 160 | 170 | 180 |
| VIETEKTLYL | IMEYASGGEV | FDYLVAHGRM | KEKEARAKFR | QIVSAVQYCH | QKRIVHRDLK |
| 190 | 200 | 210 | 220 | 230 | 240 |
| AENLLLDADM | NIKITDFGFS | NEFTVGSKLD | TFCGSPPYAA | PELFQGKKYD | GPEVDVWSLG |
| 250 | 260 | 270 | 280 | 290 | 300 |
| VILYTLVSGS | LPFDGQNLKE | LRERVLRGKY | RIPFYMSTDC | ENLLKRFLVL | NPVKRGTLEQ |
| 310 | 320 | 330 | 340 | 350 | 360 |
| IMKDRWINAG | HEEEELKPFV | EPELDISDQK | RIDIMVGMGY | SQEEIQESLS | KMKYDEITAT |
| 370 | 380 | 390 | 400 | 410 | 420 |
| YLLLGRKSAE | LDASDSSSSS | NLSLAKVRPS | SDLSNSTGQS | PHHKGQRSVS | SSQKQRRYSD |
| 430 | 440 | 450 | 460 | 470 | 480 |
| HAGPAIPSVV | AYPKRSQTST | ADSDLKEDGV | PSRKSGSSAV | GGKGIAPASP | MLGNASNPNK |
| 490 | 500 | 510 | 520 | 530 | 540 |
| ADIPERKKSP | AVPSSNAASG | GMTRRNTYVC | SERCAADRHS | VIQNGKESSL | TEVFAYAASP |
| 550 | 560 | 570 | 580 | 590 | 600 |
| ASLCATSTCR | LRHQRSMSVS | ASGHPKMVLP | PIDSEGDTFK | AITTPDQRTP | VASTHSISSA |
| 610 | 620 | 630 | 640 | 650 | 660 |
| TTPDRIRFPR | GTASRSTFHG | QPRERRTATY | NGPPASPSLS | HEATPLSQTR | SRGSTNLFSK |
| 670 | 680 | 690 | 700 | 710 | 720 |
| LTSKLTRRLP | TEYERNGRYE | GSSRNVSSEQ | KDENREAKPR | SLRFTWSMKT | TSSMDPSDMM |
| 730 | 740 | 750 | 760 | 770 | 780 |
| REIRKVLDAN | TCDYEQRERF | LLFCVHGDGH | AESLVQWEME | VCKLPRLSLN | GVRFKRISGT |
| 790 | |||||
| SIAFKNIASK | IANELKL |