Q8K1B8
Gene name |
Fermt3 (Kind3, Urp2) |
Protein name |
Fermitin family homolog 3 |
Names |
Kindlin-3 , Unc-112-related protein 2 |
Species |
Mus musculus (Mouse) |
KEGG Pathway |
mmu:108101 |
EC number |
|
Protein Class |
|
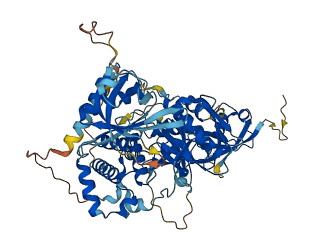
Descriptions
Autoinhibitory domains (AIDs)
Target domain |
567-665 (F3 subdomain) |
Relief mechanism |
PTM |
Assay |
|
Accessory elements
No accessory elements
Autoinhibited structure

Activated structure

2 structures for Q8K1B8
| Entry ID | Method | Resolution | Chain | Position | Source |
|---|---|---|---|---|---|
| 5L81 | X-ray | 223 A | A/B | 344-478 | PDB |
| AF-Q8K1B8-F1 | Predicted | AlphaFoldDB |
27 variants for Q8K1B8
| Variant ID(s) | Position | Change | Description | Diseaes Association | Provenance |
|---|---|---|---|---|---|
| rs3389522097 | 45 | V>A | No | EVA | |
| rs3389522438 | 77 | H>L | No | EVA | |
| rs3389534494 | 93 | F>L | No | EVA | |
| rs36766675 | 108 | R>C | No | EVA | |
| rs3389470452 | 122 | Q>K | No | EVA | |
| rs3389524133 | 182 | P>S | No | EVA | |
| rs3389524084 | 189 | A>P | No | EVA | |
| rs3409106305 | 199 | S>C | No | EVA | |
| rs3389517327 | 199 | S>N | No | EVA | |
| rs3389515792 | 207 | P>S | No | EVA | |
| rs3389470413 | 290 | E>K | No | EVA | |
| rs3389479315 | 297 | L>R | No | EVA | |
| rs3389522457 | 302 | N>S | No | EVA | |
| rs3389470485 | 314 | A>D | No | EVA | |
| rs3389522122 | 318 | L>P | No | EVA | |
| rs3389522481 | 347 | T>I | No | EVA | |
| rs30715023 | 347 | T>S | No | EVA | |
| rs217069448 | 353 | K>R | No | EVA | |
| rs3389489826 | 381 | L>P | No | EVA | |
| rs3389538002 | 384 | Y>H | No | EVA | |
| rs3389522132 | 392 | G>R | No | EVA | |
| rs3389470406 | 401 | K>T | No | EVA | |
| rs3389488040 | 423 | P>S | No | EVA | |
| rs3389534532 | 447 | A>V | No | EVA | |
| rs252331448 | 495 | P>S | No | EVA | |
| rs3389515839 | 504 | L>P | No | EVA | |
| rs3389527892 | 550 | D>N | No | EVA |
No associated diseases with Q8K1B8
5 regional properties for Q8K1B8
Functions
3 GO annotations of cellular component
| Name | Definition |
|---|---|
| cell projection | A prolongation or process extending from a cell, e.g. a flagellum or axon. |
| cell-substrate junction | A cell junction that forms a connection between a cell and the extracellular matrix. |
| podosome | An actin-rich adhesion structure characterized by formation upon cell substrate contact and localization at the substrate-attached part of the cell, contain an F-actin-rich core surrounded by a ring structure containing proteins such as vinculin and talin, and have a diameter of 0.5 mm. |
1 GO annotations of molecular function
| Name | Definition |
|---|---|
| integrin binding | Binding to an integrin. |
8 GO annotations of biological process
| Name | Definition |
|---|---|
| cell-matrix adhesion | The binding of a cell to the extracellular matrix via adhesion molecules. |
| integrin activation | The aggregation, arrangement and bonding together of an integrin, a heterodimeric adhesion receptor formed by the non-covalent association of particular alpha and beta subunits, that lead to the increased affinity of the integrin for its extracellular ligands. |
| integrin-mediated signaling pathway | The series of molecular signals initiated by an extracellular ligand binding to an integrin on the surface of a target cell, and ending with the regulation of a downstream cellular process, e.g. transcription. |
| leukocyte cell-cell adhesion | The attachment of a leukocyte to another cell via adhesion molecules. |
| platelet aggregation | The adhesion of one platelet to one or more other platelets via adhesion molecules. |
| positive regulation of cell migration | Any process that activates or increases the frequency, rate or extent of cell migration. |
| regulation of cell-cell adhesion mediated by integrin | Any process that modulates the frequency, rate, or extent of cell-cell adhesion mediated by integrin. |
| substrate adhesion-dependent cell spreading | The morphogenetic process that results in flattening of a cell as a consequence of its adhesion to a substrate. |
12 homologous proteins in AiPD
| UniProt AC | Gene Name | Protein Name | Species | Evidence Code |
|---|---|---|---|---|
| Q32LP0 | FERMT3 | Fermitin family homolog 3 | Bos taurus (Bovine) | SS |
| Q9VZI3 | Fit1 | Unc-112-related protein | Drosophila melanogaster (Fruit fly) | SS |
| Q96AC1 | FERMT2 | Fermitin family homolog 2 | Homo sapiens (Human) | SS |
| Q9BQL6 | FERMT1 | Fermitin family homolog 1 | Homo sapiens (Human) | SS |
| Q86UX7 | FERMT3 | Fermitin family homolog 3 | Homo sapiens (Human) | EV |
| Q9Y4G6 | TLN2 | Talin-2 | Homo sapiens (Human) | SS |
| Q9Y490 | TLN1 | Talin-1 | Homo sapiens (Human) | EV |
| P59113 | Fermt1 | Fermitin family homolog 1 | Mus musculus (Mouse) | SS |
| Q8CIB5 | Fermt2 | Fermitin family homolog 2 | Mus musculus (Mouse) | SS |
| P26039 | Tln1 | Talin-1 | Mus musculus (Mouse) | EV |
| Q18685 | unc-112 | Protein unc-112 | Caenorhabditis elegans | SS |
| F1Q8X5 | fermt2 | Fermitin family homolog 2 | Danio rerio (Zebrafish) (Brachydanio rerio) | SS |
| 10 | 20 | 30 | 40 | 50 | 60 |
| MAGMKTASGD | YIDSSWELRV | FVGEEDPEAQ | SVTLRVTGES | HIGGVLLKIV | EEINRKQDWS |
| 70 | 80 | 90 | 100 | 110 | 120 |
| DHAIWWEQKR | QWLLQTHWTL | DKYGILADAR | LFFGPQHRPV | ILRLPNRRVL | RLRASFSKPL |
| 130 | 140 | 150 | 160 | 170 | 180 |
| FQTVAAICRL | LSIRHPEELS | LLRAPEKKEK | KKKEKEPEEE | VHDLTKVVLA | GGVAPTLFRG |
| 190 | 200 | 210 | 220 | 230 | 240 |
| MPAHFSDSAQ | TEACYHMLSR | PQPAPDPLLL | QRLPRPSSLP | DKTQLHSRWL | DSSRCLMQQG |
| 250 | 260 | 270 | 280 | 290 | 300 |
| IKAGDVLWLR | FKYYSFFDLD | PKTDPVRLTQ | LYEQARWDLL | TEEIDCTEEE | MMVFAALQYH |
| 310 | 320 | 330 | 340 | 350 | 360 |
| INKLTLSGDV | GELASGDLGL | DDLDAALNNL | EVKLKGSAPS | DMLDSLTTIP | ELKDHLRIFR |
| 370 | 380 | 390 | 400 | 410 | 420 |
| PRKLTLKGYR | QYWVVFKDTT | LSYYKSQDEA | PGDPTQQLNL | KGCEVVPDVN | VSGQKFCIKL |
| 430 | 440 | 450 | 460 | 470 | 480 |
| LVPSPEGMSE | IYLRCQDEQQ | YAQWMAACRL | ASKGRTMADS | SYASEVQAIL | AFLSLQRAGG |
| 490 | 500 | 510 | 520 | 530 | 540 |
| SNGGSGNKPQ | GPEAPAEGLN | PYGLVAPRFQ | RKFKAKQLTP | RILEAHQNVA | QLSLTEAQLR |
| 550 | 560 | 570 | 580 | 590 | 600 |
| FIQAWQSLPD | FGISYVMVRF | KGSRKDEILG | IANNRLIRID | LAVGDVVKTW | RFSNMRQWNV |
| 610 | 620 | 630 | 640 | 650 | 660 |
| NWDIRQVAIE | FDEHINVAFS | CVSASCRIVH | EYIGGYIFLS | TRERARGEEL | DEDLFLQLTG |
| GHEAF |