Q7YQL3
Gene name |
PAK3 |
Protein name |
Serine/threonine-protein kinase PAK 3 |
Names |
Beta-PAK, p21-activated kinase 3, PAK-3 |
Species |
Pongo pygmaeus (Bornean orangutan) |
KEGG Pathway |
|
EC number |
2.7.11.1: Protein-serine/threonine kinases |
Protein Class |
|
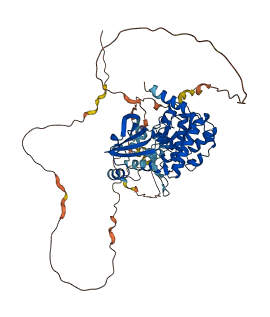
Descriptions
Autoinhibitory domains (AIDs)
Target domain |
283-534 (Protein kinase domain) |
Relief mechanism |
Partner binding |
Assay |
|
Accessory elements
419-442 (Activation loop from InterPro)
Target domain |
283-534 (Protein kinase domain) |
Relief mechanism |
|
Assay |
|
References
Autoinhibited structure

Activated structure

1 structures for Q7YQL3
| Entry ID | Method | Resolution | Chain | Position | Source |
|---|---|---|---|---|---|
| AF-Q7YQL3-F1 | Predicted | AlphaFoldDB |
No variants for Q7YQL3
| Variant ID(s) | Position | Change | Description | Diseaes Association | Provenance |
|---|---|---|---|---|---|
| No variants for Q7YQL3 | |||||
No associated diseases with Q7YQL3
4 regional properties for Q7YQL3
Functions
| Description | ||
|---|---|---|
| EC Number | 2.7.11.1 | Protein-serine/threonine kinases |
| Subcellular Localization |
|
|
| PANTHER Family | ||
| PANTHER Subfamily | ||
| PANTHER Protein Class | ||
| PANTHER Pathway Category | No pathway information available | |
1 GO annotations of cellular component
| Name | Definition |
|---|---|
| cytoplasm | The contents of a cell excluding the plasma membrane and nucleus, but including other subcellular structures. |
6 GO annotations of molecular function
| Name | Definition |
|---|---|
| ATP binding | Binding to ATP, adenosine 5'-triphosphate, a universally important coenzyme and enzyme regulator. |
| MAP kinase kinase activity | Catalysis of the concomitant phosphorylation of threonine (T) and tyrosine (Y) residues in a Thr-Glu-Tyr (TEY) thiolester sequence in a MAP kinase (MAPK) substrate. |
| metal ion binding | Binding to a metal ion. |
| protein serine kinase activity | Catalysis of the reactions: ATP + protein serine = ADP + protein serine phosphate. |
| protein serine/threonine kinase activity | Catalysis of the reactions: ATP + protein serine = ADP + protein serine phosphate, and ATP + protein threonine = ADP + protein threonine phosphate. |
| SH3 domain binding | Binding to a SH3 domain (Src homology 3) of a protein, small protein modules containing approximately 50 amino acid residues found in a great variety of intracellular or membrane-associated proteins. |
4 GO annotations of biological process
| Name | Definition |
|---|---|
| axonogenesis | De novo generation of a long process of a neuron, including the terminal branched region. Refers to the morphogenesis or creation of shape or form of the developing axon, which carries efferent (outgoing) action potentials from the cell body towards target cells. |
| dendrite development | The process whose specific outcome is the progression of the dendrite over time, from its formation to the mature structure. |
| dendritic spine morphogenesis | The process in which the anatomical structures of a dendritic spine are generated and organized. A dendritic spine is a protrusion from a dendrite and a specialized subcellular compartment involved in synaptic transmission. |
| regulation of actin filament polymerization | Any process that modulates the frequency, rate or extent of the assembly of actin filaments by the addition of actin monomers to a filament. |
No homologous proteins in AiPD
| UniProt AC | Gene Name | Protein Name | Species | Evidence Code |
|---|---|---|---|---|
| No homologous proteins | ||||
| 10 | 20 | 30 | 40 | 50 | 60 |
| MSDGLDNEEK | PPAPPLRMNS | NNRDSSALNH | SSKPLPMAPE | EKNKKARLRS | IFPGGGDKTN |
| 70 | 80 | 90 | 100 | 110 | 120 |
| KKKEKERPEI | SLPSDFEHTI | HVGFDAVTGE | FTPDLYGSQM | CPGKLPEGIP | EQWARLLQTS |
| 130 | 140 | 150 | 160 | 170 | 180 |
| NITKLEQKKN | PQAVLDVLKF | YDSKETVNNQ | KYMSFTSGDK | SAHGYIAAHP | SSTKTASEPP |
| 190 | 200 | 210 | 220 | 230 | 240 |
| LAPPVSEEED | EEEEEEEDEN | EPPPVIAPRP | EHTKSIYTRS | VVESIASPAV | PNKEVTPPSA |
| 250 | 260 | 270 | 280 | 290 | 300 |
| ENANSSTLYR | NTDRQRKKSK | MTDEEILEKL | RSIVSVGDPK | KKYTRFEKIG | QGASGTVYTA |
| 310 | 320 | 330 | 340 | 350 | 360 |
| LDIATGQEVA | IKQMNLQQQP | KKELIINEIL | VMRENKNPNI | VNYLDSYLVG | DELWVVMEYL |
| 370 | 380 | 390 | 400 | 410 | 420 |
| AGGSLTDVVT | ETCMDEGQIA | AVCRECLQAL | DFLHSNQVIH | RDIKSDNILL | GMDGSVKLTD |
| 430 | 440 | 450 | 460 | 470 | 480 |
| FGFCAQITPE | QSKRSTMVGT | PYWMAPEVVT | RKAYGPKVDI | WSLGIMAIEM | VEGEPPYLNE |
| 490 | 500 | 510 | 520 | 530 | 540 |
| NPLRALYLIA | TNGTPELQNP | ERLSAVFRDF | LNRCLEMDVD | RRGSAKELLQ | HPFLKLAKPL |
| 550 | |||||
| SSLTPLIIAA | KEAIKNSSR |