Q72L95
Gene name |
sigA |
Protein name |
RNA polymerase sigma factor SigA |
Names |
|
Species |
Thermus thermophilus (strain ATCC BAA-163 / DSM 7039 / HB27) |
KEGG Pathway |
tth:TT_C0164 |
EC number |
|
Protein Class |
RNA POLYMERASE SIGMA FACTOR RPO (PTHR30603) |
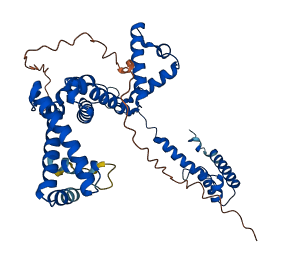
Descriptions
The DNA-dependent RNA polymerase (RNAP) is the principal enzyme of the transcription process, and is a final target in many regulatory pathways that control gene expression in all living organisms. In bacteria, RNAP is responsible for the synthesis of all RNAs in the cell (messenger RNA, ribosomal RNA, transfer RNA, and so on). The bacterial RNAP exists in two forms: core and holoenzyme. The core enzyme has a relative molecular mass of about 400,000, and consists of five subunits: α-dimer (α2), β, β' and ω. They are evolutionarily conserved in sequence, structure and function from bacteria to humans. The binding of initiation factor σ to a RNAP core enzyme results in the formation of a holoenzyme, the active form of RNAP essential for transcription initiation. The atomic model of the σ70 subunit lacks the disordered N-terminal domain (σ1-73), which includes the conserved σ region 1.1. This is a self-inhibitory domain, which is known to mask the DNA-binding regions of σ before it binds the core.
Autoinhibitory domains (AIDs)
Target domain |
187-256 (Sigma-70 factor domain-2); 357-409 (Sigma-70 factor domain-4) |
Relief mechanism |
Partner binding |
Assay |
Mutagenesis experiment, Deletion assay, Structural analysis |
Accessory elements
No accessory elements
Autoinhibited structure
Activated structure

3 structures for Q72L95
| Entry ID | Method | Resolution | Chain | Position | Source |
|---|---|---|---|---|---|
| 1IW7 | X-ray | 260 A | F/P | 1-423 | PDB |
| 1SMY | X-ray | 270 A | F/P | 1-423 | PDB |
| AF-Q72L95-F1 | Predicted | AlphaFoldDB |
No variants for Q72L95
| Variant ID(s) | Position | Change | Description | Diseaes Association | Provenance |
|---|---|---|---|---|---|
| No variants for Q72L95 | |||||
No associated diseases with Q72L95
10 regional properties for Q72L95
| Type | Name | Position | InterPro Accession |
|---|---|---|---|
| domain | RNA polymerase sigma-70 | 211 - 224 | IPR000943-1 |
| domain | RNA polymerase sigma-70 | 235 - 243 | IPR000943-2 |
| domain | RNA polymerase sigma-70 | 361 - 373 | IPR000943-3 |
| domain | RNA polymerase sigma-70 | 382 - 408 | IPR000943-4 |
| domain | RNA polymerase sigma-70 region 3 | 266 - 344 | IPR007624 |
| domain | RNA polymerase sigma-70 region 2 | 187 - 256 | IPR007627 |
| domain | RNA polymerase sigma-70 region 4 | 357 - 409 | IPR007630 |
| domain | RNA polymerase sigma-70 region 1.2 | 79 - 112 | IPR009042 |
| domain | RNA polymerase sigma factor RpoD, C-terminal | 183 - 422 | IPR012760 |
| domain | RNA polymerase sigma-70 like domain | 183 - 409 | IPR014284 |
Functions
| Description | ||
|---|---|---|
| EC Number | ||
| Subcellular Localization |
|
|
| PANTHER Family | PTHR30603 | RNA POLYMERASE SIGMA FACTOR RPO |
| PANTHER Subfamily | PTHR30603:SF47 | RNA POLYMERASE SIGMA FACTOR SIGF, CHLOROPLASTIC |
| PANTHER Protein Class |
helix-turn-helix transcription factor
Sigma factor |
|
| PANTHER Pathway Category | No pathway information available | |
1 GO annotations of cellular component
| Name | Definition |
|---|---|
| cytoplasm | The contents of a cell excluding the plasma membrane and nucleus, but including other subcellular structures. |
2 GO annotations of molecular function
| Name | Definition |
|---|---|
| DNA binding | Any molecular function by which a gene product interacts selectively and non-covalently with DNA (deoxyribonucleic acid). |
| sigma factor activity | Sigma factors act as the promoter specificity subunit of eubacterial and plant plastid multisubunit RNA polymerases, whose core subunit composition is often described as alpha(2)-beta-beta-prime. Although sigma does not bind DNA on its own, when combined with the core to form the holoenzyme, the sigma factor binds specifically to promoter elements. The sigma subunit is released once elongation begins. |
1 GO annotations of biological process
| Name | Definition |
|---|---|
| DNA-templated transcription, initiation | The initial step of transcription, consisting of the assembly of the RNA polymerase preinitiation complex (PIC) at a gene promoter, as well as the formation of the first few bonds of the RNA transcript. Transcription initiation includes abortive initiation events, which occur when the first few nucleotides are repeatedly synthesized and then released, and ends when promoter clearance takes place. |
No homologous proteins in AiPD
| UniProt AC | Gene Name | Protein Name | Species | Evidence Code |
|---|---|---|---|---|
| No homologous proteins | ||||
| 10 | 20 | 30 | 40 | 50 | 60 |
| MKKSKRKNAQ | AQEAQETEVL | VQEEAEELPE | FPEGEPDPDL | EDPDLALEDD | LLDLPEEGEG |
| 70 | 80 | 90 | 100 | 110 | 120 |
| LDLEEEEEDL | PIPKISTSDP | VRQYLHEIGQ | VPLLTLEEEV | ELARKVEEGM | EAIKKLSEIT |
| 130 | 140 | 150 | 160 | 170 | 180 |
| GLDPDLIREV | VRAKILGSAR | VRHIPGLKET | LDPKTVEEID | QKLKSLPKEH | KRYLHIAREG |
| 190 | 200 | 210 | 220 | 230 | 240 |
| EAARQHLIEA | NLRLVVSIAK | KYTGRGLSFL | DLIQEGNQGL | IRAVEKFEYK | RRFKFSTYAT |
| 250 | 260 | 270 | 280 | 290 | 300 |
| WWIRQAINRA | IADQARTIRI | PVHMVETINK | LSRTARQLQQ | ELGREPTYEE | IAEAMGPGWD |
| 310 | 320 | 330 | 340 | 350 | 360 |
| AKRVEETLKI | AQEPVSLETP | IGDEKDSFYG | DFIPDEHLPS | PVDAATQSLL | SEELEKALSK |
| 370 | 380 | 390 | 400 | 410 | 420 |
| LSEREAMVLK | LRKGLIDGRE | HTLEEVGAFF | GVTRERIRQI | ENKALRKLKY | HESRTRKLRD |
| FLD |