Q65CL1
Gene name |
Ctnna3 |
Protein name |
Catenin alpha-3 |
Names |
Alpha T-catenin, Cadherin-associated protein |
Species |
Mus musculus (Mouse) |
KEGG Pathway |
mmu:216033 |
EC number |
|
Protein Class |
ALPHA CATENIN (PTHR18914) |
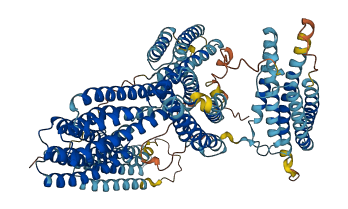
Descriptions
Catenin alpha-3 (Ctnna3, αT-catenin) functions as a crucial molecular bridge, connecting cadherin proteins to the actin cytoskeleton and ensuring the integrity of cell-cell adhesion. In contrast to Ctnna1, the αT-catenin N-terminus is required to maintain the middle (M)-region autoinhibition and modulate vinculin binding. The steric, nonspecific interactions between the αT-catenin N-terminus and M-region promote MI folding and M-region autoinhibition. Actin binding relieves the autoinhibition, which is tension-dependent.
Autoinhibitory domains (AIDs)
Target domain |
260-626 (Middle region) |
Relief mechanism |
Others |
Assay |
Deletion assay |
Accessory elements
No accessory elements
References
- Yeon JH et al. (2016) "Systems-wide Identification of cis-Regulatory Elements in Proteins", Cell systems, 2, 89-100
- Heier JA et al. (2021) "Distinct intramolecular interactions regulate autoinhibition of vinculin binding in αT-catenin and αE-catenin", The Journal of biological chemistry, 296, 100582
- Ishiyama N et al. (2013) "An autoinhibited structure of α-catenin and its implications for vinculin recruitment to adherens junctions", The Journal of biological chemistry, 288, 15913-25
- Hirano Y et al. (2018) "The force-sensing device region of α-catenin is an intrinsically disordered segment in the absence of intramolecular stabilization of the autoinhibitory form", Genes to cells : devoted to molecular & cellular mechanisms, 23, 370-385
- Choi HJ et al. (2012) "αE-catenin is an autoinhibited molecule that coactivates vinculin", Proceedings of the National Academy of Sciences of the United States of America, 109, 8576-81
- Rangarajan ES et al. (2023) "Distinct inter-domain interactions of dimeric versus monomeric α-catenin link cell junctions to filaments", Communications biology, 6, 276
- Barrick S et al. (2018) "Salt bridges gate α-catenin activation at intercellular junctions", Molecular biology of the cell, 29, 111-122
- Li J et al. (2015) "Structural Determinants of the Mechanical Stability of α-Catenin", The Journal of biological chemistry, 290, 18890-903
Autoinhibited structure

Activated structure

1 structures for Q65CL1
| Entry ID | Method | Resolution | Chain | Position | Source |
|---|---|---|---|---|---|
| AF-Q65CL1-F1 | Predicted | AlphaFoldDB |
45 variants for Q65CL1
| Variant ID(s) | Position | Change | Description | Diseaes Association | Provenance |
|---|---|---|---|---|---|
| rs3389097457 | 10 | N>K | No | EVA | |
| rs3389107742 | 25 | K>T | No | EVA | |
| rs3401331968 | 63 | E>Q | No | EVA | |
| rs3400867290 | 114 | Y>C | No | EVA | |
| rs3401331954 | 117 | K>R* | No | EVA | |
| rs3400867247 | 121 | V>G | No | EVA | |
| rs3389103530 | 137 | V>I | No | EVA | |
| rs3400614975 | 138 | L>I | No | EVA | |
| rs3401245585 | 138 | L>P | No | EVA | |
| rs3389078255 | 223 | S>A | No | EVA | |
| rs3389102534 | 251 | V>A | No | EVA | |
| rs3389102549 | 275 | S>G | No | EVA | |
| rs216723148 | 277 | F>I | No | EVA | |
| rs216723148 | 277 | F>L | No | EVA | |
| rs1133875150 | 290 | T>S | No | EVA | |
| rs1132385900 | 296 | V>L | No | EVA | |
| rs248562796 | 299 | S>L | No | EVA | |
| rs1135131131 | 303 | R>H | No | EVA | |
| rs1131789230 | 307 | I>L | No | EVA | |
| rs1134478589 | 308 | I>F | No | EVA | |
| rs3389107725 | 322 | D>Y | No | EVA | |
| rs3389078234 | 343 | L>P | No | EVA | |
| rs250165314 | 348 | S>R | No | EVA | |
| rs3389083113 | 354 | E>* | No | EVA | |
| rs3389078220 | 361 | T>S | No | EVA | |
| rs1131964311 | 375 | R>K | No | EVA | |
| rs1133021921 | 376 | Q>H | No | EVA | |
| rs3389048155 | 385 | I>V | No | EVA | |
| rs3389102614 | 415 | A>D | No | EVA | |
| rs3401300809 | 423 | G>C | No | EVA | |
| rs3401399693 | 423 | G>D | No | EVA | |
| rs3401239291 | 426 | V>L | No | EVA | |
| rs3389114771 | 430 | N>Y | No | EVA | |
| rs3389107698 | 435 | M>I | No | EVA | |
| rs3412866419 | 461 | N>Y | No | EVA | |
| rs46078493 | 535 | R>Q | No | EVA | |
| rs3389091155 | 536 | A>T | No | EVA | |
| rs3389109742 | 593 | L>V | No | EVA | |
| rs3401138689 | 610 | S>F | No | EVA | |
| rs3400873924 | 611 | K>* | No | EVA | |
| rs3401246884 | 611 | K>M | No | EVA | |
| rs3400873939 | 612 | K>E | No | EVA | |
| rs3389113892 | 638 | D>N | No | EVA | |
| rs3389091071 | 814 | L>S | No | EVA | |
| rs3389109744 | 817 | A>V | No | EVA |
No associated diseases with Q65CL1
No regional properties for Q65CL1
| Type | Name | Position | InterPro Accession |
|---|---|---|---|
| No domain, repeats, and functional sites for Q65CL1 | |||
Functions
| Description | ||
|---|---|---|
| EC Number | ||
| Subcellular Localization |
|
|
| PANTHER Family | PTHR18914 | ALPHA CATENIN |
| PANTHER Subfamily | PTHR18914:SF21 | CATENIN ALPHA-3 |
| PANTHER Protein Class | non-motor actin binding protein | |
| PANTHER Pathway Category |
Wnt signaling pathway alpha-catenin Cadherin signaling pathway alpha-catenin |
|
5 GO annotations of cellular component
| Name | Definition |
|---|---|
| adherens junction | A cell-cell junction composed of the epithelial cadherin-catenin complex. The epithelial cadherins, or E-cadherins, of each interacting cell extend through the plasma membrane into the extracellular space and bind to each other. The E-cadherins bind to catenins on the cytoplasmic side of the membrane, where the E-cadherin-catenin complex binds to cytoskeletal components and regulatory and signaling molecules. |
| cytoskeleton | A cellular structure that forms the internal framework of eukaryotic and prokaryotic cells. The cytoskeleton includes intermediate filaments, microfilaments, microtubules, the microtrabecular lattice, and other structures characterized by a polymeric filamentous nature and long-range order within the cell. The various elements of the cytoskeleton not only serve in the maintenance of cellular shape but also have roles in other cellular functions, including cellular movement, cell division, endocytosis, and movement of organelles. |
| cytosol | The part of the cytoplasm that does not contain organelles but which does contain other particulate matter, such as protein complexes. |
| fascia adherens | A cell-cell junction that contains the transmembrane protein N-cadherin, which interacts with identical molecules from neighbouring cells to form a tight mechanical intercellular link; forms a large portion of the intercalated disc, the structure at which myofibrils terminate in cardiomyocytes. |
| lamellipodium | A thin sheetlike process extended by the leading edge of a migrating cell or extending cell process; contains a dense meshwork of actin filaments. |
3 GO annotations of molecular function
| Name | Definition |
|---|---|
| actin filament binding | Binding to an actin filament, also known as F-actin, a helical filamentous polymer of globular G-actin subunits. |
| beta-catenin binding | Binding to a catenin beta subunit. |
| cadherin binding | Binding to cadherin, a type I membrane protein involved in cell adhesion. |
7 GO annotations of biological process
| Name | Definition |
|---|---|
| actin filament organization | A process that is carried out at the cellular level which results in the assembly, arrangement of constituent parts, or disassembly of cytoskeletal structures comprising actin filaments. Includes processes that control the spatial distribution of actin filaments, such as organizing filaments into meshworks, bundles, or other structures, as by cross-linking. |
| bundle of His cell-Purkinje myocyte adhesion involved in cell communication | The attachment of a bundle of His cell to a Purkinje myocyte via adhesion molecules that results in the cells being juxtaposed so that they can communicate. |
| cell migration | The controlled self-propelled movement of a cell from one site to a destination guided by molecular cues. Cell migration is a central process in the development and maintenance of multicellular organisms. |
| cell-cell adhesion | The attachment of one cell to another cell via adhesion molecules. |
| epithelial cell-cell adhesion | The attachment of an epithelial cell to another epithelial cell via adhesion molecules. |
| regulation of heart rate by cardiac conduction | A cardiac conduction process that modulates the frequency or rate of heart contraction. |
| regulation of ventricular cardiac muscle cell action potential | Any process that modulates the frequency, rate or extent of action potential creation, propagation or termination in a ventricular cardiac muscle cell contributing to the regulation of its contraction. This typically occurs via modulation of the activity or expression of voltage-gated ion channels. |
16 homologous proteins in AiPD
| UniProt AC | Gene Name | Protein Name | Species | Evidence Code |
|---|---|---|---|---|
| Q3MHM6 | CTNNA1 | Catenin alpha-1 | Bos taurus (Bovine) | SS |
| P12003 | VCL | Vinculin | Gallus gallus (Chicken) | EV |
| P30997 | CTNNA2 | Catenin alpha-2 | Gallus gallus (Chicken) | SS |
| P35220 | alpha-Cat | Catenin alpha | Drosophila melanogaster (Fruit fly) | SS |
| P18206 | VCL | Vinculin | Homo sapiens (Human) | SS |
| P26232 | CTNNA2 | Catenin alpha-2 | Homo sapiens (Human) | SS |
| P35221 | CTNNA1 | Catenin alpha-1 | Homo sapiens (Human) | EV |
| Q9UI47 | CTNNA3 | Catenin alpha-3 | Homo sapiens (Human) | SS |
| Q64727 | Vcl | Vinculin | Mus musculus (Mouse) | SS |
| P26231 | Ctnna1 | Catenin alpha-1 | Mus musculus (Mouse) | EV |
| Q61301 | Ctnna2 | Catenin alpha-2 | Mus musculus (Mouse) | EV |
| P26234 | VCL | Vinculin | Sus scrofa (Pig) | SS |
| P85972 | Vcl | Vinculin | Rattus norvegicus (Rat) | SS |
| P90947 | hmp-1 | Alpha-catenin-like protein hmp-1 | Caenorhabditis elegans | SS |
| A4IGI7 | ctnna2 | Catenin alpha-2 | Xenopus tropicalis (Western clawed frog) (Silurana tropicalis) | SS |
| B7ZC77 | Ctnna2 | Catenin alpha-2 | Danio rerio (Zebrafish) (Brachydanio rerio) | SS |
| 10 | 20 | 30 | 40 | 50 | 60 |
| MSAETPITLN | MDTQDLQIQT | FTVEKLLEPL | IIQVTTLVNC | PQNPSNRKKG | RSKRARVLLA |
| 70 | 80 | 90 | 100 | 110 | 120 |
| SVEEATWNLL | DKGEMIAKEA | TVLKEELAAA | LQEVRKESKA | LKVSAERFTD | DPCYLPKREA |
| 130 | 140 | 150 | 160 | 170 | 180 |
| VVQAARALLA | AVTRLLVLAD | MIDVMCLLQH | VSSFQRTFES | LKNVSNKSDL | QRTYQKLGKE |
| 190 | 200 | 210 | 220 | 230 | 240 |
| LESLDYLAFK | RQQDLKSPSQ | RDEIAGARAT | LKENSPLLHS | ICSACLEHSD | VASLKASKDT |
| 250 | 260 | 270 | 280 | 290 | 300 |
| VCEEIQNALD | VISNASQGIQ | NAPAPPEPQA | ATLGSAFDEL | ENLIVLNPLT | VTEEDVRPSL |
| 310 | 320 | 330 | 340 | 350 | 360 |
| EKRLEAIISG | AALLADSSCT | RDLHRERIIA | ECNAIRQALQ | DLLTEYMSNT | GKTERSNTLN |
| 370 | 380 | 390 | 400 | 410 | 420 |
| TAIVNMSKKT | RDLRRQLRKA | IIDHISDSFL | DTTVPLLVLI | EAAKNGRVKE | IKDYAAIFHE |
| 430 | 440 | 450 | 460 | 470 | 480 |
| HTGRLVEVAN | LACSMSTNED | GIKIVRIAAN | HLETLCPQII | NAALALASRP | KSQVVKNTME |
| 490 | 500 | 510 | 520 | 530 | 540 |
| MYKRTWEHYI | HVLTEAVDDI | TSIDDFLAVS | ESHILEDVNK | CIIALRDQDA | DNLDRAAGAI |
| 550 | 560 | 570 | 580 | 590 | 600 |
| RGRAARVAHI | VAGEMDSYEP | GAYTEGVMRN | VNFLTSTVIP | EFVTQVNVAL | DALSKNSLTA |
| 610 | 620 | 630 | 640 | 650 | 660 |
| LDDNQFVDIS | KKIYDTIHDI | RCSVMMIRTP | EELEDVSDLE | DDHEVRSHTS | IQTEGKTDRA |
| 670 | 680 | 690 | 700 | 710 | 720 |
| KMTQLPEAEK | EKIAEQVADF | KKVKSKLDAE | IEIWDDTSND | IIVLAKKMCM | IMMEMTDFTR |
| 730 | 740 | 750 | 760 | 770 | 780 |
| GKGPLKHTTD | VIYAAKMISE | SGSRMDVLAR | QIANQCPDPP | CKQDLLAYLE | QIKFYSHQLK |
| 790 | 800 | 810 | 820 | 830 | 840 |
| ICSQVKAEIQ | NLGGELIVSA | LDSVTSLIQA | AKNLMNAVVQ | TVKMSYIAST | KIIRIQSSAG |
| 850 | 860 | 870 | 880 | 890 | |
| PRHPVVMWRM | KAPAKKPLIK | REKPEETWAA | VRRGSAKKKI | HPVQVMSEFR | GRQVY |