Q5ZL74
Gene name |
VAMP7 (SYBL1, RCJMB04_7f19) |
Protein name |
Vesicle-associated membrane protein 7 |
Names |
Synaptobrevin-like protein 1 |
Species |
Gallus gallus (Chicken) |
KEGG Pathway |
gga:422297 |
EC number |
|
Protein Class |
|
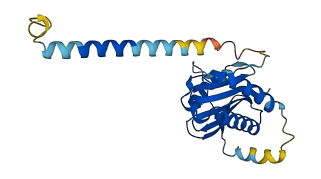
Descriptions
Autoinhibitory domains (AIDs)
Target domain |
125-185 (v-SNARE coiled-coil homology) |
Relief mechanism |
|
Assay |
|
Accessory elements
No accessory elements
References
- Yeon JH et al. (2016) "Systems-wide Identification of cis-Regulatory Elements in Proteins", Cell systems, 2, 89-100
- Martinez-Arca S et al. (2003) "A dual mechanism controlling the localization and function of exocytic v-SNAREs", Proceedings of the National Academy of Sciences of the United States of America, 100, 9011-6
- Gonzalez LC Jr et al. (2001) "A novel snare N-terminal domain revealed by the crystal structure of Sec22b", The Journal of biological chemistry, 276, 24203-11
- Mancias JD et al. (2007) "The transport signal on Sec22 for packaging into COPII-coated vesicles is a conformational epitope", Molecular cell, 26, 403-14
- Wen W et al. (2010) "Lipid-Induced conformational switch controls fusion activity of longin domain SNARE Ykt6", Molecular cell, 37, 383-95
Autoinhibited structure

Activated structure

1 structures for Q5ZL74
| Entry ID | Method | Resolution | Chain | Position | Source |
|---|---|---|---|---|---|
| AF-Q5ZL74-F1 | Predicted | AlphaFoldDB |
No variants for Q5ZL74
| Variant ID(s) | Position | Change | Description | Diseaes Association | Provenance |
|---|---|---|---|---|---|
| No variants for Q5ZL74 | |||||
No associated diseases with Q5ZL74
Functions
10 GO annotations of cellular component
| Name | Definition |
|---|---|
| endoplasmic reticulum membrane | The lipid bilayer surrounding the endoplasmic reticulum. |
| Golgi apparatus | A membrane-bound cytoplasmic organelle of the endomembrane system that further processes the core oligosaccharides (e.g. N-glycans) added to proteins in the endoplasmic reticulum and packages them into membrane-bound vesicles. The Golgi apparatus operates at the intersection of the secretory, lysosomal, and endocytic pathways. |
| late endosome membrane | The lipid bilayer surrounding a late endosome. |
| lysosomal membrane | The lipid bilayer surrounding the lysosome and separating its contents from the cell cytoplasm. |
| neuron projection | A prolongation or process extending from a nerve cell, e.g. an axon or dendrite. |
| phagocytic vesicle | A membrane-bounded intracellular vesicle that arises from the ingestion of particulate material by phagocytosis. |
| phagocytic vesicle membrane | The lipid bilayer surrounding a phagocytic vesicle. |
| SNARE complex | A protein complex involved in membrane fusion; a stable ternary complex consisting of a four-helix bundle, usually formed from one R-SNARE and three Q-SNAREs with an ionic layer sandwiched between hydrophobic layers. One well-characterized example is the neuronal SNARE complex formed of synaptobrevin 2, syntaxin 1a, and SNAP-25. |
| synapse | The junction between an axon of one neuron and a dendrite of another neuron, a muscle fiber or a glial cell. As the axon approaches the synapse it enlarges into a specialized structure, the presynaptic terminal bouton, which contains mitochondria and synaptic vesicles. At the tip of the terminal bouton is the presynaptic membrane; facing it, and separated from it by a minute cleft (the synaptic cleft) is a specialized area of membrane on the receiving cell, known as the postsynaptic membrane. In response to the arrival of nerve impulses, the presynaptic terminal bouton secretes molecules of neurotransmitters into the synaptic cleft. These diffuse across the cleft and transmit the signal to the postsynaptic membrane. |
| transport vesicle membrane | The lipid bilayer surrounding a transport vesicle. |
2 GO annotations of molecular function
| Name | Definition |
|---|---|
| SNAP receptor activity | Acting as a marker to identify a membrane and interacting selectively with one or more SNAREs on another membrane to mediate membrane fusion. |
| SNARE binding | Binding to a SNARE (soluble N-ethylmaleimide-sensitive factor attached protein receptor) protein. |
10 GO annotations of biological process
| Name | Definition |
|---|---|
| calcium-ion regulated exocytosis | The release of intracellular molecules (e.g. hormones, matrix proteins) contained within a membrane-bounded vesicle by fusion of the vesicle with the plasma membrane of a cell, induced by a rise in cytosolic calcium-ion levels. |
| endoplasmic reticulum to Golgi vesicle-mediated transport | The directed movement of substances from the endoplasmic reticulum (ER) to the Golgi, mediated by COP II vesicles. Small COP II coated vesicles form from the ER and then fuse directly with the cis-Golgi. Larger structures are transported along microtubules to the cis-Golgi. |
| endosome to lysosome transport | The directed movement of substances from endosomes to lysosomes. |
| eosinophil degranulation | The regulated exocytosis of secretory granules containing preformed mediators such as major basic protein, eosinophil peroxidase, and eosinophil cationic protein by an eosinophil. |
| exocytosis | A process of secretion by a cell that results in the release of intracellular molecules (e.g. hormones, matrix proteins) contained within a membrane-bounded vesicle. Exocytosis can occur either by full fusion, when the vesicle collapses into the plasma membrane, or by a kiss-and-run mechanism that involves the formation of a transient contact, a pore, between a granule (for exemple of chromaffin cells) and the plasma membrane. The latter process most of the time leads to only partial secretion of the granule content. Exocytosis begins with steps that prepare vesicles for fusion with the membrane (tethering and docking) and ends when molecules are secreted from the cell. |
| neutrophil degranulation | The regulated exocytosis of secretory granules containing preformed mediators such as proteases, lipases, and inflammatory mediators by a neutrophil. |
| phagocytosis, engulfment | The internalization of bacteria, immune complexes and other particulate matter or of an apoptotic cell by phagocytosis, including the membrane and cytoskeletal processes required, which involves one of three mechanisms |
| protein transport | The directed movement of proteins into, out of or within a cell, or between cells, by means of some agent such as a transporter or pore. |
| vesicle fusion | Fusion of the membrane of a transport vesicle with its target membrane. |
| vesicle-mediated transport | A cellular transport process in which transported substances are moved in membrane-bounded vesicles; transported substances are enclosed in the vesicle lumen or located in the vesicle membrane. The process begins with a step that directs a substance to the forming vesicle, and includes vesicle budding and coating. Vesicles are then targeted to, and fuse with, an acceptor membrane. |
10 homologous proteins in AiPD
| UniProt AC | Gene Name | Protein Name | Species | Evidence Code |
|---|---|---|---|---|
| Q12255 | NYV1 | Vacuolar v-SNARE NYV1 | Saccharomyces cerevisiae (strain ATCC 204508 / S288c) (Baker's yeast) | PR |
| Q17QI5 | VAMP7 | Vesicle-associated membrane protein 7 | Bos taurus (Bovine) | SS |
| P51809 | VAMP7 | Vesicle-associated membrane protein 7 | Homo sapiens (Human) | EV |
| P70280 | Vamp7 | Vesicle-associated membrane protein 7 | Mus musculus (Mouse) | SS |
| Q9JHW5 | Vamp7 | Vesicle-associated membrane protein 7 | Rattus norvegicus (Rat) | SS |
| O49377 | VAMP711 | Vesicle-associated membrane protein 711 | Arabidopsis thaliana (Mouse-ear cress) | SS |
| Q9FMR5 | VAMP714 | Vesicle-associated membrane protein 714 | Arabidopsis thaliana (Mouse-ear cress) | PR |
| Q9LFP1 | VAMP713 | Vesicle-associated membrane protein 713 | Arabidopsis thaliana (Mouse-ear cress) | SS |
| Q9M376 | VAMP727 | Vesicle-associated membrane protein 727 | Arabidopsis thaliana (Mouse-ear cress) | SS |
| Q9SIQ9 | VAMP712 | Vesicle-associated membrane protein 712 | Arabidopsis thaliana (Mouse-ear cress) | SS |
| 10 | 20 | 30 | 40 | 50 | 60 |
| MAILFAVVAR | GTTILAKHAW | CGGNFLEVTE | QILAKIPSEN | NKLTYSHGNY | LFHYICQDRI |
| 70 | 80 | 90 | 100 | 110 | 120 |
| IYLCITDDDF | ERSRAFNFLN | EIKKRFQTTY | GSRAQTALPY | AMNSEFSSVL | AAQLKYHSES |
| 130 | 140 | 150 | 160 | 170 | 180 |
| KGTDQVAETQ | AQVDELKGIM | VRNIDLVAQR | GEKLELLIDK | TENLVDSSVT | FKTTSRNLAR |
| 190 | 200 | 210 | |||
| AMCMKNLKLT | IIIIIVSIVI | IYIIVSAACG | GLAWPSCVQK |