Q32L27
Gene name |
UBE2Q2 |
Protein name |
Ubiquitin-conjugating enzyme E2 Q2 |
Names |
E2 ubiquitin-conjugating enzyme Q2, Ubiquitin carrier protein Q2, Ubiquitin-protein ligase Q2 |
Species |
Bos taurus (Bovine) |
KEGG Pathway |
bta:511631 |
EC number |
2.3.2.23: Aminoacyltransferases |
Protein Class |
|
Descriptions
The autoinhibited protein was predicted that may have potential autoinhibitory elements via cis-regPred.
Autoinhibitory domains (AIDs)
Target domain |
|
Relief mechanism |
|
Assay |
cis-regPred |
Accessory elements
No accessory elements
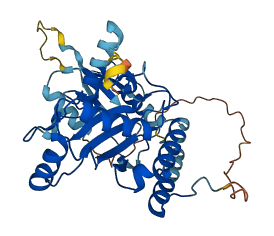
Autoinhibited structure

Activated structure

1 structures for Q32L27
| Entry ID | Method | Resolution | Chain | Position | Source |
|---|---|---|---|---|---|
| AF-Q32L27-F1 | Predicted | AlphaFoldDB |
51 variants for Q32L27
| Variant ID(s) | Position | Change | Description | Diseaes Association | Provenance |
|---|---|---|---|---|---|
| rs434312964 | 3 | V>G | No | EVA | |
| rs473025730 | 9 | E>A | No | EVA | |
| rs441703368 | 9 | E>D | No | EVA | |
| rs475562834 | 12 | F>L | No | EVA | |
| rs461878736 | 12 | F>V | No | EVA | |
| rs444230435 | 14 | A>E | No | EVA | |
| rs457906637 | 15 | S>A | No | EVA | |
| rs457906637 | 15 | S>P | No | EVA | |
| rs440293539 | 16 | I>M | No | EVA | |
| rs460362921 | 24 | F>L | No | EVA | |
| rs469509326 | 41 | P>T | No | EVA | |
| rs483218979 | 43 | P>T | No | EVA | |
| rs465592100 | 47 | G>A | No | EVA | |
| rs132738989 | 48 | S>I | No | EVA | |
| rs132738989 | 48 | S>T | No | EVA | |
| rs134935455 | 50 | H>L | No | EVA | |
| rs134935455 | 50 | H>P | No | EVA | |
| rs134935455 | 50 | H>R | No | EVA | |
| rs136326618 | 51 | S>A | No | EVA | |
| rs136326618 | 51 | S>P | No | EVA | |
| rs475535882 | 53 | P>A | No | EVA | |
| rs444141767 | 56 | L>R | No | EVA | |
| rs471445660 | 58 | L>P | No | EVA | |
| rs471445660 | 58 | L>R | No | EVA | |
| rs440230116 | 59 | H>P | No | EVA | |
| rs440230116 | 59 | H>R | No | EVA | |
| rs460276952 | 60 | C>G | No | EVA | |
| rs480377883 | 60 | C>S | No | EVA | |
| rs442800218 | 61 | N>H | No | EVA | |
| rs462954268 | 61 | N>S | No | EVA | |
| rs483143734 | 62 | I>L | No | EVA | |
| rs445324747 | 63 | T>R | No | EVA | |
| rs448236961 | 64 | E>D | No | EVA | |
| rs379986206 | 90 | E>Q | No | EVA | |
| rs458402636 | 126 | M>I | No | EVA | |
| rs478730970 | 129 | E>G | No | EVA | |
| rs209572777 | 132 | I>V | No | EVA | |
| rs481264909 | 143 | E>* | No | EVA | |
| rs449965299 | 152 | K>* | No | EVA | |
| rs432920919 | 173 | S>* | No | EVA | |
| rs473188507 | 174 | D>V | No | EVA | |
| rs461894327 | 186 | S>* | No | EVA | |
| rs441879465 | 186 | S>A | No | EVA | |
| rs444377136 | 187 | Q>R | No | EVA | |
| rs482468012 | 279 | G>R | No | EVA | |
| rs451109455 | 280 | W>C | No | EVA | |
| rs464824320 | 288 | S>L | No | EVA | |
| rs451760727 | 311 | N>H | No | EVA | |
| rs471916592 | 312 | Q>K | No | EVA | |
| rs440638547 | 323 | N>S | No | EVA | |
| rs460782934 | 324 | S>P | No | EVA |
No associated diseases with Q32L27
1 regional properties for Q32L27
| Type | Name | Position | InterPro Accession |
|---|---|---|---|
| domain | Ubiquitin-conjugating enzyme E2 | 171 - 335 | IPR000608 |
Functions
| Description | ||
|---|---|---|
| EC Number | 2.3.2.23 | Aminoacyltransferases |
| Subcellular Localization |
|
|
| PANTHER Family | ||
| PANTHER Subfamily | ||
| PANTHER Protein Class | ||
| PANTHER Pathway Category | No pathway information available | |
2 GO annotations of cellular component
| Name | Definition |
|---|---|
| cytoplasm | The contents of a cell excluding the plasma membrane and nucleus, but including other subcellular structures. |
| nucleus | A membrane-bounded organelle of eukaryotic cells in which chromosomes are housed and replicated. In most cells, the nucleus contains all of the cell's chromosomes except the organellar chromosomes, and is the site of RNA synthesis and processing. In some species, or in specialized cell types, RNA metabolism or DNA replication may be absent. |
3 GO annotations of molecular function
| Name | Definition |
|---|---|
| ATP binding | Binding to ATP, adenosine 5'-triphosphate, a universally important coenzyme and enzyme regulator. |
| ubiquitin conjugating enzyme activity | Isoenergetic transfer of ubiquitin from one protein to another via the reaction X-ubiquitin + Y -> Y-ubiquitin + X, where both the X-ubiquitin and Y-ubiquitin linkages are thioester bonds between the C-terminal glycine of ubiquitin and a sulfhydryl side group of a cysteine residue. |
| ubiquitin-protein transferase activity | Catalysis of the transfer of ubiquitin from one protein to another via the reaction X-Ub + Y --> Y-Ub + X, where both X-Ub and Y-Ub are covalent linkages. |
2 GO annotations of biological process
| Name | Definition |
|---|---|
| protein K48-linked ubiquitination | A protein ubiquitination process in which a polymer of ubiquitin, formed by linkages between lysine residues at position 48 of the ubiquitin monomers, is added to a protein. K48-linked ubiquitination targets the substrate protein for degradation. |
| protein polyubiquitination | Addition of multiple ubiquitin groups to a protein, forming a ubiquitin chain. |
8 homologous proteins in AiPD
| UniProt AC | Gene Name | Protein Name | Species | Evidence Code |
|---|---|---|---|---|
| P61085 | UBE2K | Ubiquitin-conjugating enzyme E2 K | Bos taurus (Bovine) | PR |
| Q32LD2 | UBE2T | Ubiquitin-conjugating enzyme E2 T | Bos taurus (Bovine) | PR |
| Q32PA5 | UBE2C | Ubiquitin-conjugating enzyme E2 C | Bos taurus (Bovine) | PR |
| Q1RML1 | UBE2S | Ubiquitin-conjugating enzyme E2 S | Bos taurus (Bovine) | SS |
| Q7Z7E8 | UBE2Q1 | Ubiquitin-conjugating enzyme E2 Q1 | Homo sapiens (Human) | PR |
| A1L167 | UBE2QL1 | Ubiquitin-conjugating enzyme E2Q-like protein 1 | Homo sapiens (Human) | PR |
| A0PJN4 | Ube2ql1 | Ubiquitin-conjugating enzyme E2Q-like protein 1 | Mus musculus (Mouse) | PR |
| Q7TSS2 | Ube2q1 | Ubiquitin-conjugating enzyme E2 Q1 | Mus musculus (Mouse) | PR |
| 10 | 20 | 30 | 40 | 50 | 60 |
| MSVSGLKAEL | KFLASIFDKN | HERFRIVSWK | LDELHCQFLV | PPPAPPGSPH | SPPPPLTLHC |
| 70 | 80 | 90 | 100 | 110 | 120 |
| NITESYPSSS | PIWFVDSDDP | NLTSVLERLE | DTKNNNSNGT | TEEVTSEEEE | EEEMAEDIED |
| 130 | 140 | 150 | 160 | 170 | 180 |
| LDHYEMKEEE | PISGKKSEDE | GIEKENLAIL | EKIRKTQRQD | HLNGAVSGSV | QASDRLMKEL |
| 190 | 200 | 210 | 220 | 230 | 240 |
| RDIYRSQSYK | TGIYSVELIN | DSLYDWHVKL | QKVDPDSPLH | SDLQILKEKE | GIEYILLNFS |
| 250 | 260 | 270 | 280 | 290 | 300 |
| FKDNFPFDPP | FVRVVLPVLS | GGYVLGGGAL | CMELLTKQGW | SSAYSIESVI | MQINATLVKG |
| 310 | 320 | 330 | 340 | ||
| KARVQFGANK | NQYNLARAQQ | SYNSIVQIHE | KNGWYTPPKE | DG |