Q1ZXK2
Gene name |
forG |
Protein name |
Formin-G |
Names |
|
Species |
Dictyostelium discoideum (Slime mold) |
KEGG Pathway |
ddi:DDB_G0277175 |
EC number |
|
Protein Class |
|
Descriptions
Autoinhibitory domains (AIDs)
Target domain |
631-1072 (FH2 domain) |
Relief mechanism |
Partner binding |
Assay |
|
Target domain |
631-1072 (FH2 domain) |
Relief mechanism |
Partner binding |
Assay |
|
Accessory elements
No accessory elements
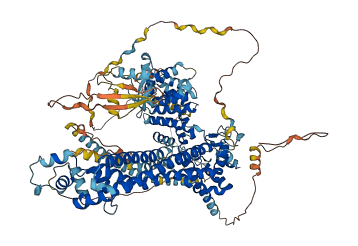
Autoinhibited structure

Activated structure

1 structures for Q1ZXK2
| Entry ID | Method | Resolution | Chain | Position | Source |
|---|---|---|---|---|---|
| AF-Q1ZXK2-F1 | Predicted | AlphaFoldDB |
No variants for Q1ZXK2
| Variant ID(s) | Position | Change | Description | Diseaes Association | Provenance |
|---|---|---|---|---|---|
| No variants for Q1ZXK2 | |||||
No associated diseases with Q1ZXK2
4 GO annotations of cellular component
| Name | Definition |
|---|---|
| cytoplasm | The contents of a cell excluding the plasma membrane and nucleus, but including other subcellular structures. |
| cytoskeleton | A cellular structure that forms the internal framework of eukaryotic and prokaryotic cells. The cytoskeleton includes intermediate filaments, microfilaments, microtubules, the microtrabecular lattice, and other structures characterized by a polymeric filamentous nature and long-range order within the cell. The various elements of the cytoskeleton not only serve in the maintenance of cellular shape but also have roles in other cellular functions, including cellular movement, cell division, endocytosis, and movement of organelles. |
| macropinocytic cup cytoskeleton | The part of the cortical actin cytoskeleton that forms part of a macropinocytic cup. |
| phagocytic cup | An invagination of the cell membrane formed by an actin dependent process during phagocytosis. Following internalization it is converted into a phagosome. |
2 GO annotations of molecular function
| Name | Definition |
|---|---|
| actin filament binding | Binding to an actin filament, also known as F-actin, a helical filamentous polymer of globular G-actin subunits. |
| profilin binding | Binding to profilin, an actin-binding protein that forms a complex with G-actin and prevents it from polymerizing to form F-actin. |
5 GO annotations of biological process
| Name | Definition |
|---|---|
| actin filament network formation | The assembly of a network of actin filaments; actin filaments on different axes and with differing orientations are crosslinked together to form a mesh of filaments. |
| cortical actin cytoskeleton organization | A process that is carried out at the cellular level which results in the assembly, arrangement of constituent parts, or disassembly of actin-based cytoskeletal structures in the cell cortex, i.e. just beneath the plasma membrane. |
| positive regulation of actin filament polymerization | Any process that activates or increases the frequency, rate or extent of actin polymerization. |
| positive regulation of macropinocytosis | Any process that activates or increases the frequency, rate or extent of macropinocytosis. |
| positive regulation of phagocytosis, engulfment | Any process that activates or increases the frequency, rate or extent of the internalization of bacteria, immune complexes and other particulate matter or of an apoptotic cell by phagocytosis. |
No homologous proteins in AiPD
| UniProt AC | Gene Name | Protein Name | Species | Evidence Code |
|---|---|---|---|---|
| No homologous proteins | ||||
| 10 | 20 | 30 | 40 | 50 | 60 |
| MILSITFQLD | PISNNSTSLS | NSIDNRSSIN | VTALQMQQGS | KTYNLDQNSS | YKTVFLAICQ |
| 70 | 80 | 90 | 100 | 110 | 120 |
| LFGIKESSAQ | DYCLQLESSK | KYLNWPPQSD | ESKTKHIVKQ | LYESGETALV | LQLNPTYRSS |
| 130 | 140 | 150 | 160 | 170 | 180 |
| KWVKALNDSE | TNEKDIMFHL | KYKLQENEFA | ESFIEQNGME | GILRMVTNGK | GNAQTYSLAS |
| 190 | 200 | 210 | 220 | 230 | 240 |
| LRACLEYVSA | MEIITKTPHL | VKQLFSLVDS | NVVGVCRGAL | ELLFVLCDFR | KEEGFKSVHL |
| 250 | 260 | 270 | 280 | 290 | 300 |
| AAKGTAVAQG | KKPYLNVIKL | LDSGDLETKI | NAFTLLNVLL | SNCPTDEKVG | KLCKKWGELG |
| 310 | 320 | 330 | 340 | 350 | 360 |
| LDDKLRSLTS | IQQQEFQIQL | EIYEETSGVN | LRTKASRLEA | ICNRLKSKLT | EYEAQQPLIA |
| 370 | 380 | 390 | 400 | 410 | 420 |
| ILKEELKLAQ | QLIKEASTDR | VFLSSHPMQR | YLGPTIQSYP | ADLSFLKTTA | AERDKINEFE |
| 430 | 440 | 450 | 460 | 470 | 480 |
| KKILAINEQL | RQETKLSEEL | KNQVLANKKQ | FDSTIAELNE | ENQRLTQVEV | KFKLQQSISP |
| 490 | 500 | 510 | 520 | 530 | 540 |
| SQDSSNNQKA | SSSSSNTSTL | NDSDIQSIQS | SLKEATLEIE | RLKLAIEEKM | PPNEAQQIFS |
| 550 | 560 | 570 | 580 | 590 | 600 |
| QSLITTAAFT | PTSPDISNDG | QPISGGGAPP | PSPSPPPPIS | GGGAPPPPPP | PPPPPSGGGA |
| 610 | 620 | 630 | 640 | 650 | 660 |
| PPPPPPPPPS | GGKKAGAPGA | PPTGPAAIQP | NKPVINPSSK | MKPLYWKRII | LPPSNRNESI |
| 670 | 680 | 690 | 700 | 710 | 720 |
| WDQVLEPTFD | SKDFENLFCA | KKKAVDSSLS | TNPSSTTGKE | GEKVKLVSLV | DIKKSNSIAF |
| 730 | 740 | 750 | 760 | 770 | 780 |
| MLAKIPTAEG | LKKAIDTVDN | SILGKEIIKT | LITNVPTEQD | YQLIKGSEIH | ESKLDKPERW |
| 790 | 800 | 810 | 820 | 830 | 840 |
| ILEIYGFPMM | KERLVAWLFQ | LEYQEMYNNI | IQILEKLQNA | IKDTKSSDSL | KKILGIVLVL |
| 850 | 860 | 870 | 880 | 890 | 900 |
| GNYMNGGSGR | GQADGFTLEI | LDSLATSKDV | ENKTSLLDYV | SKISMEKYPK | TMNVAQELDS |
| 910 | 920 | 930 | 940 | 950 | 960 |
| LKLVQLSISD | MSTDINDLEK | QFNISKNNCK | KVLEANIPSS | SKFQSTIGSF | LEKTEIDIKN |
| 970 | 980 | 990 | 1000 | 1010 | 1020 |
| LKENQKNIVD | SFIQLVEFFG | YPKSYATTAS | CQQFFNSIYS | FSLLFSKQCQ | KIEKEREALA |
| 1030 | 1040 | 1050 | 1060 | 1070 | |
| KASGDNGAVQ | NKKIAGGADP | LAALANAIKL | GQTGLRKRPG | PENSSGGSQL | NLNK |