Q1XH17
Gene name |
Trim72 |
Protein name |
Tripartite motif-containing protein 72 |
Names |
Mitsugumin-53, Mg53 |
Species |
Mus musculus (Mouse) |
KEGG Pathway |
mmu:434246 |
EC number |
|
Protein Class |
|
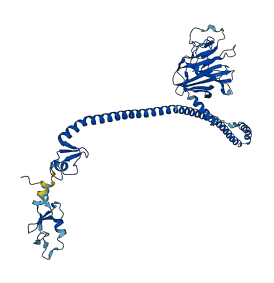
Descriptions
The autoinhibited protein was predicted that may have potential autoinhibitory elements via cis-regPred.
Autoinhibitory domains (AIDs)
Target domain |
|
Relief mechanism |
|
Assay |
cis-regPred |
Accessory elements
No accessory elements
Autoinhibited structure

Activated structure

7 structures for Q1XH17
23 variants for Q1XH17
| Variant ID(s) | Position | Change | Description | Diseaes Association | Provenance |
|---|---|---|---|---|---|
| rs3388946493 | 33 | F>L | No | EVA | |
| rs3398617584 | 42 | A>E | No | EVA | |
| rs3388950329 | 102 | T>I | No | EVA | |
| rs3388931453 | 108 | C>* | No | EVA | |
| rs33182050 | 126 | Q>H | No | EVA | |
| rs3388931483 | 146 | R>C | No | EVA | |
| rs229541754 | 150 | T>S | No | EVA | |
| rs3398544563 | 156 | H>P | No | EVA | |
| rs3388931529 | 162 | E>* | No | EVA | |
| rs3388908254 | 212 | L>Q | No | EVA | |
| rs3388946533 | 215 | Y>* | No | EVA | |
| rs3388923927 | 230 | A>T | No | EVA | |
| rs3388950566 | 298 | A>E | No | EVA | |
| rs3398130882 | 378 | Q>PL* | No | EVA | |
| rs33189379 | 385 | L>V | No | EVA | |
| rs3388946525 | 399 | E>K | No | EVA | |
| rs3388950572 | 410 | P>Q | No | EVA | |
| rs3388958121 | 418 | S>C | No | EVA | |
| rs3388955968 | 418 | S>N | No | EVA | |
| rs3388940387 | 431 | N>K | No | EVA | |
| rs3388954643 | 436 | T>M | No | EVA | |
| rs3398535066 | 475 | E>D | No | EVA | |
| rs220771768 | 477 | A>S | No | EVA |
No associated diseases with Q1XH17
10 regional properties for Q1XH17
| Type | Name | Position | InterPro Accession |
|---|---|---|---|
| domain | B-box-type zinc finger | 81 - 122 | IPR000315 |
| domain | Zinc finger, RING-type | 14 - 57 | IPR001841 |
| domain | B30.2/SPRY domain | 271 - 475 | IPR001870 |
| domain | SPRY domain | 342 - 473 | IPR003877 |
| domain | Butyrophylin-like, SPRY domain | 287 - 304 | IPR003879-1 |
| domain | Butyrophylin-like, SPRY domain | 327 - 351 | IPR003879-2 |
| domain | Butyrophylin-like, SPRY domain | 357 - 370 | IPR003879-3 |
| domain | Butyrophylin-like, SPRY domain | 404 - 428 | IPR003879-4 |
| domain | SPRY-associated | 288 - 341 | IPR006574 |
| conserved_site | Zinc finger, RING-type, conserved site | 29 - 38 | IPR017907 |
Functions
3 GO annotations of cellular component
| Name | Definition |
|---|---|
| cytoplasm | The contents of a cell excluding the plasma membrane and nucleus, but including other subcellular structures. |
| cytoplasmic vesicle membrane | The lipid bilayer surrounding a cytoplasmic vesicle. |
| sarcolemma | The outer membrane of a muscle cell, consisting of the plasma membrane, a covering basement membrane (about 100 nm thick and sometimes common to more than one fiber), and the associated loose network of collagen fibers. |
5 GO annotations of molecular function
| Name | Definition |
|---|---|
| identical protein binding | Binding to an identical protein or proteins. |
| phosphatidylserine binding | Binding to phosphatidylserine, a class of glycophospholipids in which a phosphatidyl group is esterified to the hydroxyl group of L-serine. |
| ubiquitin conjugating enzyme binding | Binding to a ubiquitin conjugating enzyme, any of the E2 proteins. |
| ubiquitin protein ligase activity | Catalysis of the transfer of ubiquitin to a substrate protein via the reaction X-ubiquitin + S -> X + S-ubiquitin, where X is either an E2 or E3 enzyme, the X-ubiquitin linkage is a thioester bond, and the S-ubiquitin linkage is an amide bond: an isopeptide bond between the C-terminal glycine of ubiquitin and the epsilon-amino group of lysine residues in the substrate or, in the linear extension of ubiquitin chains, a peptide bond the between the C-terminal glycine and N-terminal methionine of ubiquitin residues. |
| zinc ion binding | Binding to a zinc ion (Zn). |
11 GO annotations of biological process
| Name | Definition |
|---|---|
| exocytosis | A process of secretion by a cell that results in the release of intracellular molecules (e.g. hormones, matrix proteins) contained within a membrane-bounded vesicle. Exocytosis can occur either by full fusion, when the vesicle collapses into the plasma membrane, or by a kiss-and-run mechanism that involves the formation of a transient contact, a pore, between a granule (for exemple of chromaffin cells) and the plasma membrane. The latter process most of the time leads to only partial secretion of the granule content. Exocytosis begins with steps that prepare vesicles for fusion with the membrane (tethering and docking) and ends when molecules are secreted from the cell. |
| muscle organ development | The process whose specific outcome is the progression of the muscle over time, from its formation to the mature structure. The muscle is an organ consisting of a tissue made up of various elongated cells that are specialized to contract and thus to produce movement and mechanical work. |
| muscle system process | A organ system process carried out at the level of a muscle. Muscle tissue is composed of contractile cells or fibers. |
| negative regulation of insulin receptor signaling pathway | Any process that stops, prevents, or reduces the frequency, rate or extent of insulin receptor signaling. |
| negative regulation of insulin-like growth factor receptor signaling pathway | Any process that stops, prevents, or reduces the frequency, rate or extent of insulin-like growth factor receptor signaling. |
| negative regulation of myotube differentiation | Any process that decreases the frequency, rate or extent of myotube differentiation. Myotube differentiation is the process in which a relatively unspecialized cell acquires specialized features of a myotube cell. Myotubes are multinucleated cells that are formed when proliferating myoblasts exit the cell cycle, differentiate and fuse. |
| plasma membrane repair | The resealing of a cell plasma membrane after cellular wounding due to, for instance, mechanical stress. |
| proteasome-mediated ubiquitin-dependent protein catabolic process | The chemical reactions and pathways resulting in the breakdown of a protein or peptide by hydrolysis of its peptide bonds, initiated by the covalent attachment of ubiquitin, and mediated by the proteasome. |
| protein homooligomerization | The process of creating protein oligomers, compounds composed of a small number, usually between three and ten, of identical component monomers. Oligomers may be formed by the polymerization of a number of monomers or the depolymerization of a large protein polymer. |
| protein ubiquitination | The process in which one or more ubiquitin groups are added to a protein. |
| vesicle budding from membrane | The evagination of a membrane, resulting in formation of a vesicle. |
46 homologous proteins in AiPD
| UniProt AC | Gene Name | Protein Name | Species | Evidence Code |
|---|---|---|---|---|
| Q5E9G4 | TRIM10 | Tripartite motif-containing protein 10 | Bos taurus (Bovine) | PR |
| Q2T9Z0 | TRIM17 | E3 ubiquitin-protein ligase TRIM17 | Bos taurus (Bovine) | PR |
| E1BJS7 | TRIM71 | E3 ubiquitin-protein ligase TRIM71 | Bos taurus (Bovine) | PR |
| Q7YRV4 | TRIM21 | E3 ubiquitin-protein ligase TRIM21 | Bos taurus (Bovine) | PR |
| Q1PRL4 | TRIM71 | E3 ubiquitin-protein ligase TRIM71 | Gallus gallus (Chicken) | PR |
| Q7YR32 | TRIM10 | Tripartite motif-containing protein 10 | Pan troglodytes (Chimpanzee) | PR |
| O15553 | MEFV | Pyrin | Homo sapiens (Human) | SS |
| Q9BTV5 | FSD1 | Fibronectin type III and SPRY domain-containing protein 1 | Homo sapiens (Human) | PR |
| Q9H2S5 | RNF39 | RING finger protein 39 | Homo sapiens (Human) | PR |
| P19474 | TRIM21 | E3 ubiquitin-protein ligase TRIM21 | Homo sapiens (Human) | PR |
| Q9UJV3 | MID2 | Probable E3 ubiquitin-protein ligase MID2 | Homo sapiens (Human) | PR |
| P29590 | PML | Protein PML | Homo sapiens (Human) | PR |
| Q9C029 | TRIM7 | E3 ubiquitin-protein ligase TRIM7 | Homo sapiens (Human) | PR |
| Q9NQ86 | TRIM36 | E3 ubiquitin-protein ligase TRIM36 | Homo sapiens (Human) | PR |
| Q86UV6 | TRIM74 | Tripartite motif-containing protein 74 | Homo sapiens (Human) | PR |
| Q9UPQ4 | TRIM35 | E3 ubiquitin-protein ligase TRIM35 | Homo sapiens (Human) | PR |
| Q86UV7 | TRIM73 | Tripartite motif-containing protein 73 | Homo sapiens (Human) | PR |
| Q8N9V2 | TRIML1 | Probable E3 ubiquitin-protein ligase TRIML1 | Homo sapiens (Human) | PR |
| Q86XT4 | TRIM50 | E3 ubiquitin-protein ligase TRIM50 | Homo sapiens (Human) | PR |
| Q5EBN2 | TRIM61 | Putative tripartite motif-containing protein 61 | Homo sapiens (Human) | PR |
| Q9BZY9 | TRIM31 | E3 ubiquitin-protein ligase TRIM31 | Homo sapiens (Human) | PR |
| Q2Q1W2 | TRIM71 | E3 ubiquitin-protein ligase TRIM71 | Homo sapiens (Human) | PR |
| Q9BXM9 | FSD1L | FSD1-like protein | Homo sapiens (Human) | PR |
| Q6ZMU5 | TRIM72 | Tripartite motif-containing protein 72 | Homo sapiens (Human) | PR |
| Q8BZT2 | Sh3rf2 | E3 ubiquitin-protein ligase SH3RF2 | Mus musculus (Mouse) | PR |
| Q60953 | Pml | Protein PML | Mus musculus (Mouse) | PR |
| Q99PQ1 | Trim12a | Tripartite motif-containing protein 12A | Mus musculus (Mouse) | PR |
| Q61510 | Trim25 | E3 ubiquitin/ISG15 ligase TRIM25 | Mus musculus (Mouse) | PR |
| Q1PSW8 | Trim71 | E3 ubiquitin-protein ligase TRIM71 | Mus musculus (Mouse) | PR |
| P86449 | Trim43c | Tripartite motif-containing protein 43C | Mus musculus (Mouse) | PR |
| Q3TL54 | Trim43a | Tripartite motif-containing protein 43A | Mus musculus (Mouse) | PR |
| Q7TPM3 | Trim17 | E3 ubiquitin-protein ligase TRIM17 | Mus musculus (Mouse) | PR |
| Q810I2 | Trim50 | E3 ubiquitin-protein ligase TRIM50 | Mus musculus (Mouse) | PR |
| Q9JJ26 | Mefv | Pyrin | Mus musculus (Mouse) | SS |
| Q9WUH5 | Trim10 | Tripartite motif-containing protein 10 | Mus musculus (Mouse) | PR |
| O77666 | TRIM26 | Tripartite motif-containing protein 26 | Sus scrofa (Pig) | PR |
| O19085 | TRIM10 | Tripartite motif-containing protein 10 | Sus scrofa (Pig) | PR |
| Q865W2 | TRIM50 | E3 ubiquitin-protein ligase TRIM50 | Sus scrofa (Pig) | PR |
| Q920M2 | Rnf39 | RING finger protein 39 | Rattus norvegicus (Rat) | PR |
| Q9JJ25 | Mefv | Pyrin | Rattus norvegicus (Rat) | SS |
| Q810I1 | Trim50 | E3 ubiquitin-protein ligase TRIM50 | Rattus norvegicus (Rat) | PR |
| D3ZVM4 | Trim71 | E3 ubiquitin-protein ligase TRIM71 | Rattus norvegicus (Rat) | PR |
| A0JPQ4 | Trim72 | Tripartite motif-containing protein 72 | Rattus norvegicus (Rat) | PR |
| F6QEU4 | trim71 | E3 ubiquitin-protein ligase TRIM71 | Xenopus tropicalis (Western clawed frog) (Silurana tropicalis) | PR |
| Q640S6 | trim72 | Tripartite motif-containing protein 72 | Xenopus tropicalis (Western clawed frog) (Silurana tropicalis) | PR |
| E7FAM5 | trim71 | E3 ubiquitin-protein ligase TRIM71 | Danio rerio (Zebrafish) (Brachydanio rerio) | PR |
| 10 | 20 | 30 | 40 | 50 | 60 |
| MSAAPGLLRQ | ELSCPLCLQL | FDAPVTAECG | HSFCRACLIR | VAGEPAADGT | VACPCCQAPT |
| 70 | 80 | 90 | 100 | 110 | 120 |
| RPQALSTNLQ | LSRLVEGLAQ | VPQGHCEEHL | DPLSIYCEQD | RTLVCGVCAS | LGSHRGHRLL |
| 130 | 140 | 150 | 160 | 170 | 180 |
| PAAEAQARLK | TQLPQQKMQL | QEACMRKEKT | VAVLEHQLVE | VEETVRQFRG | AVGEQLGKMR |
| 190 | 200 | 210 | 220 | 230 | 240 |
| MFLAALESSL | DREAERVRGD | AGVALRRELS | SLNSYLEQLR | QMEKVLEEVA | DKPQTEFLMK |
| 250 | 260 | 270 | 280 | 290 | 300 |
| FCLVTSRLQK | ILSESPPPAR | LDIQLPVISD | DFKFQVWKKM | FRALMPALEE | LTFDPSSAHP |
| 310 | 320 | 330 | 340 | 350 | 360 |
| SLVVSSSGRR | VECSDQKAPP | AGEDTRQFDK | AVAVVAQQLL | SQGEHYWEVE | VGDKPRWALG |
| 370 | 380 | 390 | 400 | 410 | 420 |
| VMAADASRRG | RLHAVPSQGL | WLLGLRDGKI | LEAHVEAKEP | RALRTPERPP | ARIGLYLSFA |
| 430 | 440 | 450 | 460 | 470 | |
| DGVLAFYDAS | NPDVLTPIFS | FHERLPGPVY | PIFDVCWHDK | GKNAQPLLLV | GPEQEQA |