Q0IIR9
Gene name |
katna1 |
Protein name |
Katanin p60 ATPase-containing subunit A1 |
Names |
Katanin p60 subunit A1, p60 katanin |
Species |
Xenopus tropicalis (Western clawed frog) (Silurana tropicalis) |
KEGG Pathway |
xtr:779887 |
EC number |
5.6.1.1: Enzymes altering polypeptide conformation or assembly |
Protein Class |
|
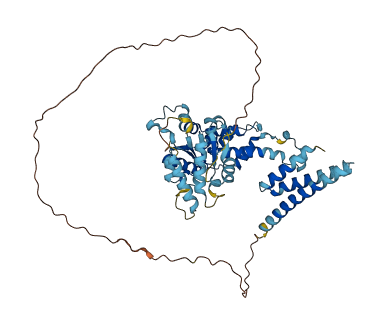
Descriptions
The autoinhibited protein was predicted that may have potential autoinhibitory elements via cis-regPred.
Autoinhibitory domains (AIDs)
Target domain |
|
Relief mechanism |
|
Assay |
cis-regPred |
Accessory elements
No accessory elements
Autoinhibited structure

Activated structure

1 structures for Q0IIR9
| Entry ID | Method | Resolution | Chain | Position | Source |
|---|---|---|---|---|---|
| AF-Q0IIR9-F1 | Predicted | AlphaFoldDB |
No variants for Q0IIR9
| Variant ID(s) | Position | Change | Description | Diseaes Association | Provenance |
|---|---|---|---|---|---|
| No variants for Q0IIR9 | |||||
No associated diseases with Q0IIR9
Functions
| Description | ||
|---|---|---|
| EC Number | 5.6.1.1 | Enzymes altering polypeptide conformation or assembly |
| Subcellular Localization |
|
|
| PANTHER Family | ||
| PANTHER Subfamily | ||
| PANTHER Protein Class | ||
| PANTHER Pathway Category | No pathway information available | |
8 GO annotations of cellular component
| Name | Definition |
|---|---|
| centrosome | A structure comprised of a core structure (in most organisms, a pair of centrioles) and peripheral material from which a microtubule-based structure, such as a spindle apparatus, is organized. Centrosomes occur close to the nucleus during interphase in many eukaryotic cells, though in animal cells it changes continually during the cell-division cycle. |
| cytoplasm | The contents of a cell excluding the plasma membrane and nucleus, but including other subcellular structures. |
| microtubule | Any of the long, generally straight, hollow tubes of internal diameter 12-15 nm and external diameter 24 nm found in a wide variety of eukaryotic cells; each consists (usually) of 13 protofilaments of polymeric tubulin, staggered in such a manner that the tubulin monomers are arranged in a helical pattern on the microtubular surface, and with the alpha/beta axes of the tubulin subunits parallel to the long axis of the tubule; exist in equilibrium with pool of tubulin monomers and can be rapidly assembled or disassembled in response to physiological stimuli; concerned with force generation, e.g. in the spindle. |
| microtubule cytoskeleton | The part of the cytoskeleton (the internal framework of a cell) composed of microtubules and associated proteins. |
| midbody | A thin cytoplasmic bridge formed between daughter cells at the end of cytokinesis. The midbody forms where the contractile ring constricts, and may persist for some time before finally breaking to complete cytokinesis. |
| mitotic spindle pole | Either of the ends of a mitotic spindle, a spindle that forms as part of mitosis, where spindle microtubules are organized; usually contains a microtubule organizing center and accessory molecules, spindle microtubules and astral microtubules. |
| spindle | The array of microtubules and associated molecules that forms between opposite poles of a eukaryotic cell during mitosis or meiosis and serves to move the duplicated chromosomes apart. |
| spindle pole | Either of the ends of a spindle, where spindle microtubules are organized; usually contains a microtubule organizing center and accessory molecules, spindle microtubules and astral microtubules. |
5 GO annotations of molecular function
| Name | Definition |
|---|---|
| ATP binding | Binding to ATP, adenosine 5'-triphosphate, a universally important coenzyme and enzyme regulator. |
| ATP hydrolysis activity | Catalysis of the reaction: ATP + H2O = ADP + H+ phosphate. ATP hydrolysis is used in some reactions as an energy source, for example to catalyze a reaction or drive transport against a concentration gradient. |
| isomerase activity | Catalysis of the geometric or structural changes within one molecule. Isomerase is the systematic name for any enzyme of EC class 5. |
| microtubule binding | Binding to a microtubule, a filament composed of tubulin monomers. |
| microtubule severing ATPase activity | Catalysis of the reaction: ATP + H2O = ADP + phosphate. Catalysis of the severing of a microtubule at a specific spot along its length, coupled to the hydrolysis of ATP. |
3 GO annotations of biological process
| Name | Definition |
|---|---|
| cell cycle | The progression of biochemical and morphological phases and events that occur in a cell during successive cell replication or nuclear replication events. Canonically, the cell cycle comprises the replication and segregation of genetic material followed by the division of the cell, but in endocycles or syncytial cells nuclear replication or nuclear division may not be followed by cell division. |
| cell division | The process resulting in division and partitioning of components of a cell to form more cells; may or may not be accompanied by the physical separation of a cell into distinct, individually membrane-bounded daughter cells. |
| microtubule severing | The process in which a microtubule is broken down into smaller segments. Severing enzymes remove dimers from the middle of the filament to create new ends, unlike depolymerizing kinesins that use ATP to uncap microtubules at their ends. |
3 homologous proteins in AiPD
| UniProt AC | Gene Name | Protein Name | Species | Evidence Code |
|---|---|---|---|---|
| O75449 | KATNA1 | Katanin p60 ATPase-containing subunit A1 | Homo sapiens (Human) | PR |
| Q8K0T4 | Katnal1 | Katanin p60 ATPase-containing subunit A-like 1 | Mus musculus (Mouse) | PR |
| A0JMA9 | katnal2 | Katanin p60 ATPase-containing subunit A-like 2 | Xenopus tropicalis (Western clawed frog) (Silurana tropicalis) | PR |
| 10 | 20 | 30 | 40 | 50 | 60 |
| MSLLMISENV | KLAREYALLG | NYDSAMVYYQ | GVLDQMNKYL | YSVKDTFLQQ | KWQQVWQEIN |
| 70 | 80 | 90 | 100 | 110 | 120 |
| MEAKHVKDIM | STLEGFKLDN | SPVKTTQHEF | PAHDGEVWSL | PVPVERRPSP | GPRKRQSVQC |
| 130 | 140 | 150 | 160 | 170 | 180 |
| NDNKSHNNRF | GAGKGPNLPS | SKNTNNVKMK | PVRAREKKDT | FLKVKDEKNK | SSVDVSETEV |
| 190 | 200 | 210 | 220 | 230 | 240 |
| KKFDGTGYDK | DLIEALERDI | ISQNPNIRWD | DIADLEEAKK | LLKEAVVLPM | WMPEFFKGIR |
| 250 | 260 | 270 | 280 | 290 | 300 |
| RPWKGVLMVG | PPGTGKTLLA | KAVATECKTT | FFNISSSTLT | SKYRGESEKL | VRLLFEMARF |
| 310 | 320 | 330 | 340 | 350 | 360 |
| YAPTTIFIDE | IDSICSRRGT | SEEHEASRRV | KAELLVQMDG | VGGASENEDP | SKMVMVLAAT |
| 370 | 380 | 390 | 400 | 410 | 420 |
| NFPWDIDEAL | RRRLEKRIYI | PLPSAKGREE | LLRINLKELE | LADDVNIECI | AENMDGYSGA |
| 430 | 440 | 450 | 460 | 470 | 480 |
| DITNVCRDAS | LMAMRRRIEG | LTPEEIRNLS | RDDMHMPTTM | EDFEMALKKV | SKSVSASDIE |
| 490 | |||||
| KYEKWIEEFG | SC |