P70704
Gene name |
Atp8a1 |
Protein name |
Phospholipid-transporting ATPase IA |
Names |
ATPase class I type 8A member 1, Chromaffin granule ATPase II, P4-ATPase flippase complex alpha subunit ATP8A1 |
Species |
Mus musculus (Mouse) |
KEGG Pathway |
mmu:11980 |
EC number |
7.6.2.1: Linked to the hydrolysis of a nucleoside triphosphate |
Protein Class |
|
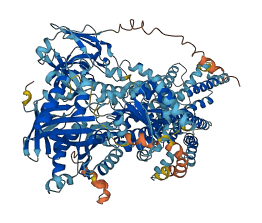
Descriptions
The autoinhibited protein was predicted that may have potential autoinhibitory elements via cis-regPred.
Autoinhibitory domains (AIDs)
Target domain |
|
Relief mechanism |
|
Assay |
cis-regPred |
Accessory elements
No accessory elements
Autoinhibited structure

Activated structure

1 structures for P70704
| Entry ID | Method | Resolution | Chain | Position | Source |
|---|---|---|---|---|---|
| AF-P70704-F1 | Predicted | AlphaFoldDB |
60 variants for P70704
| Variant ID(s) | Position | Change | Description | Diseaes Association | Provenance |
|---|---|---|---|---|---|
| rs3395325181 | 61 | V>M | No | EVA | |
| rs3388764940 | 86 | L>P | No | EVA | |
| rs3388757762 | 145 | I>T | No | EVA | |
| rs3388765512 | 189 | D>E | No | EVA | |
| rs3388752858 | 193 | N>I | No | EVA | |
| rs3388757394 | 193 | N>K | No | EVA | |
| rs3388767361 | 240 | H>R | No | EVA | |
| rs3395703424 | 253 | R>* | No | EVA | |
| rs3388765458 | 273 | D>V | No | EVA | |
| rs3395462508 | 326 | K>N | No | EVA | |
| rs3395333911 | 330 | L>H | No | EVA | |
| rs3388767425 | 425 | T>I | No | EVA | |
| rs3388760013 | 426 | I>M | No | EVA | |
| rs234738063 | 434 | V>A | No | EVA | |
| rs3388746648 | 439 | D>E | No | EVA | |
| rs3388764867 | 440 | Y>* | No | EVA | |
| rs3388757388 | 445 | D>E | No | EVA | |
| rs1132070624 | 454 | D>N | No | EVA | |
| rs1135189842 | 455 | E>V | No | EVA | |
| rs864292200 | 464 | L>P | No | EVA | |
| rs3388764596 | 468 | Q>R | No | EVA | |
| rs3388736570 | 471 | H>R | No | EVA | |
| rs3388750493 | 472 | P>L | No | EVA | |
| rs251535852 | 486 | V>I | No | EVA | |
| rs3388746690 | 521 | V>F | No | EVA | |
| rs3388736522 | 576 | T>I | No | EVA | |
| rs864262113 | 622 | W>C | No | EVA | |
| rs864305380 | 623 | R>W | No | EVA | |
| rs3388757409 | 645 | E>K | No | EVA | |
| rs3388771336 | 681 | I>L | No | EVA | |
| rs3388764588 | 683 | I>V | No | EVA | |
| rs3388736545 | 686 | G>E | No | EVA | |
| rs3388762383 | 686 | G>W | No | EVA | |
| rs3395648534 | 698 | S>T | No | EVA | |
| rs3388748627 | 703 | K>E | No | EVA | |
| rs3395705455 | 707 | G>E | No | EVA | |
| rs3388764599 | 754 | T>I | No | EVA | |
| rs3388736559 | 779 | L>F | No | EVA | |
| rs3388736542 | 794 | V>A | No | EVA | |
| rs3388748553 | 809 | I>N | No | EVA | |
| rs3388762437 | 812 | A>V | No | EVA | |
| rs3388752810 | 815 | G>S | No | EVA | |
| rs3395653027 | 864 | Y>* | No | EVA | |
| rs3395593953 | 864 | Y>F | No | EVA | |
| rs3395674164 | 865 | K>* | No | EVA | |
| rs3388746629 | 891 | W>* | No | EVA | |
| rs3388771384 | 897 | N>I | No | EVA | |
| rs3388765517 | 905 | P>T | No | EVA | |
| rs3388752866 | 943 | V>I | No | EVA | |
| rs3388771355 | 983 | F>L | No | EVA | |
| rs3388755941 | 1017 | L>P | No | EVA | |
| rs3388762045 | 1024 | I>F | No | EVA | |
| rs3388764943 | 1097 | V>L | No | EVA | |
| rs3388765143 | 1100 | G>E | No | EVA | |
| rs3395461446 | 1104 | T>S | No | EVA | |
| rs3388764889 | 1107 | A>V | No | EVA | |
| rs3388763989 | 1153 | A>S | No | EVA | |
| rs3388758508 | 1162 | D>E | No | EVA | |
| rs3388736521 | 1162 | D>V | No | EVA | |
| rs3388748580 | 1165 | W>C | No | EVA |
No associated diseases with P70704
1 regional properties for P70704
| Type | Name | Position | InterPro Accession |
|---|---|---|---|
| domain | Zinc finger, RING-type | 234 - 276 | IPR001841 |
Functions
| Description | ||
|---|---|---|
| EC Number | 7.6.2.1 | Linked to the hydrolysis of a nucleoside triphosphate |
| Subcellular Localization |
|
|
| PANTHER Family | ||
| PANTHER Subfamily | ||
| PANTHER Protein Class | ||
| PANTHER Pathway Category | No pathway information available | |
10 GO annotations of cellular component
| Name | Definition |
|---|---|
| chromaffin granule membrane | The lipid bilayer surrounding a chromaffin granule, a specialized secretory vesicle found in the cells of adrenal glands and various other organs, which is concerned with the synthesis, storage, metabolism, and secretion of epinephrine and norepinephrine. |
| cytoplasmic vesicle | A vesicle found in the cytoplasm of a cell. |
| endoplasmic reticulum | The irregular network of unit membranes, visible only by electron microscopy, that occurs in the cytoplasm of many eukaryotic cells. The membranes form a complex meshwork of tubular channels, which are often expanded into slitlike cavities called cisternae. The ER takes two forms, rough (or granular), with ribosomes adhering to the outer surface, and smooth (with no ribosomes attached). |
| Golgi apparatus | A membrane-bound cytoplasmic organelle of the endomembrane system that further processes the core oligosaccharides (e.g. N-glycans) added to proteins in the endoplasmic reticulum and packages them into membrane-bound vesicles. The Golgi apparatus operates at the intersection of the secretory, lysosomal, and endocytic pathways. |
| integral component of synaptic vesicle membrane | The component of the synaptic vesicle membrane consisting of the gene products and protein complexes having at least some part of their peptide sequence embedded in the hydrophobic region of the membrane. |
| intracellular membrane-bounded organelle | Organized structure of distinctive morphology and function, bounded by a single or double lipid bilayer membrane and occurring within the cell. Includes the nucleus, mitochondria, plastids, vacuoles, and vesicles. Excludes the plasma membrane. |
| organelle membrane | A membrane that is one of the two lipid bilayers of an organelle envelope or the outermost membrane of single membrane bound organelle. |
| phospholipid-translocating ATPase complex | A protein complex that functions as a phospholipid-translocating P-Type ATPase. |
| plasma membrane | The membrane surrounding a cell that separates the cell from its external environment. It consists of a phospholipid bilayer and associated proteins. |
| trans-Golgi network | The network of interconnected tubular and cisternal structures located within the Golgi apparatus on the side distal to the endoplasmic reticulum, from which secretory vesicles emerge. The trans-Golgi network is important in the later stages of protein secretion where it is thought to play a key role in the sorting and targeting of secreted proteins to the correct destination. |
6 GO annotations of molecular function
| Name | Definition |
|---|---|
| ATP binding | Binding to ATP, adenosine 5'-triphosphate, a universally important coenzyme and enzyme regulator. |
| ATP hydrolysis activity | Catalysis of the reaction: ATP + H2O = ADP + H+ phosphate. ATP hydrolysis is used in some reactions as an energy source, for example to catalyze a reaction or drive transport against a concentration gradient. |
| ATPase-coupled intramembrane lipid transporter activity | Catalysis of the movement of lipids from one membrane leaflet to the other, driven by ATP hydrolysis. This includes flippases and floppases. |
| magnesium ion binding | Binding to a magnesium (Mg) ion. |
| phosphatidylserine flippase activity | Catalysis of the movement of phosphatidylserine from the exoplasmic to the cytosolic leaftlet of a membrane, using energy from the hydrolysis of ATP. |
| phosphatidylserine floppase activity | Catalysis of the movement of phosphatidylserine from the cytosolic to the exoplasmic leaftlet of a membrane, using energy from the hydrolysis of ATP. |
5 GO annotations of biological process
| Name | Definition |
|---|---|
| aminophospholipid translocation | The movement of an aminophospholipid molecule from one leaflet of a membrane bilayer to the opposite leaflet. |
| learning | Any process in an organism in which a relatively long-lasting adaptive behavioral change occurs as the result of experience. |
| phospholipid translocation | The movement of a phospholipid molecule from one leaflet of a membrane bilayer to the opposite leaflet. |
| positive regulation of cell migration | Any process that activates or increases the frequency, rate or extent of cell migration. |
| positive regulation of phospholipid translocation | Any process that increases the frequency, rate or extent of the translocation, or flipping, of phospholipid molecules from one monolayer of a membrane bilayer to the opposite monolayer. |
11 homologous proteins in AiPD
| UniProt AC | Gene Name | Protein Name | Species | Evidence Code |
|---|---|---|---|---|
| P39524 | DRS2 | Phospholipid-transporting ATPase DRS2 | Saccharomyces cerevisiae (strain ATCC 204508 / S288c) (Baker's yeast) | EV |
| Q9P241 | ATP10D | Phospholipid-transporting ATPase VD | Homo sapiens (Human) | PR |
| Q8TF62 | ATP8B4 | Probable phospholipid-transporting ATPase IM | Homo sapiens (Human) | PR |
| P98198 | ATP8B2 | Phospholipid-transporting ATPase ID | Homo sapiens (Human) | PR |
| O43520 | ATP8B1 | Phospholipid-transporting ATPase IC | Homo sapiens (Human) | EV |
| Q9Y2Q0 | ATP8A1 | Phospholipid-transporting ATPase IA | Homo sapiens (Human) | PR |
| Q148W0 | Atp8b1 | Phospholipid-transporting ATPase IC | Mus musculus (Mouse) | SS |
| P98199 | Atp8b2 | Phospholipid-transporting ATPase ID | Mus musculus (Mouse) | PR |
| D4AA47 | Atp8b1 | Phospholipid-transporting ATPase IC | Rattus norvegicus (Rat) | SS |
| Q9U280 | tat-1 | Phospholipid-transporting ATPase tat-1 | Caenorhabditis elegans | PR |
| Q5BL50 | atp8b1 | Phospholipid-transporting ATPase IC | Xenopus tropicalis (Western clawed frog) (Silurana tropicalis) | SS |
| 10 | 20 | 30 | 40 | 50 | 60 |
| MPTMRRTVSE | IRSRAEGYEK | TDDVSEKTSL | ADQEEVRTIF | INQPQLTKFC | NNHVSTAKYN |
| 70 | 80 | 90 | 100 | 110 | 120 |
| VITFLPRFLY | SQFRRAANSF | FLFIALLQQI | PDVSPTGRYT | TLVPLLFILA | VAAIKEIIED |
| 130 | 140 | 150 | 160 | 170 | 180 |
| IKRHKADNAV | NKKQTQVLRN | GAWEIVHWEK | VAVGEIVKVT | NGEHLPADLL | SLSSSEPQAM |
| 190 | 200 | 210 | 220 | 230 | 240 |
| CYIETSNLDG | ETNLKIRQGL | PATSDIKDID | SLMRISGRIE | CESPNRHLYD | FVGNIRLDGH |
| 250 | 260 | 270 | 280 | 290 | 300 |
| GTVPLGADQI | LLRGAQLRNT | QWVHGIVVYT | GHDTKLMQNS | TSPPLKLSNV | ERITNVQILI |
| 310 | 320 | 330 | 340 | 350 | 360 |
| LFCILIAMSL | VCSVGSAIWN | RRHSGKDWYL | HLHYGGASNF | GLNFLTFIIL | FNNLIPISLL |
| 370 | 380 | 390 | 400 | 410 | 420 |
| VTLEVVKFTQ | AYFINWDLDM | HYEPTDTAAM | ARTSNLNEEL | GQVKYIFSDK | TGTLTCNVMQ |
| 430 | 440 | 450 | 460 | 470 | 480 |
| FKKCTIAGVA | YGHVPEPEDY | GCSPDEWQSS | QFGDEKTFND | PSLLDNLQNN | HPTAPIICEF |
| 490 | 500 | 510 | 520 | 530 | 540 |
| LTMMAVCHTA | VPEREGDKII | YQAASPDEGA | LVRAAKQLNF | VFTGRTPDSV | IIDSLGQEER |
| 550 | 560 | 570 | 580 | 590 | 600 |
| YELLNVLEFT | SARKRMSVVV | RTPSGKLRLY | CKGADTVIYE | RLAETSKYKE | ITLKHLEQFA |
| 610 | 620 | 630 | 640 | 650 | 660 |
| TEGLRTLCFA | VAEISESDFE | EWRAVYHRAS | TSVQNRLLKL | EESYELIEKN | LQLLGATAIE |
| 670 | 680 | 690 | 700 | 710 | 720 |
| DKLQDQVPET | IETLMKADIK | IWILTGDKQE | TAINIGHSCR | LLKRNMGMIV | INEGSLDGTR |
| 730 | 740 | 750 | 760 | 770 | 780 |
| ETLSRHCTTL | GDALRKENDF | ALIIDGKTLK | YALTFGVRQY | FLDLALSCKA | VICCRVSPLQ |
| 790 | 800 | 810 | 820 | 830 | 840 |
| KSEVVEMVKK | QVKVITLAIG | DGANDVSMIQ | TAHVGVGISG | NEGLQAANSS | DYSIAQFKYL |
| 850 | 860 | 870 | 880 | 890 | 900 |
| KNLLMVHGAW | NYNRVSKCIL | YCFYKNIVLY | IIEIWFAFVN | GFSGQILFER | WCIGLYNVMF |
| 910 | 920 | 930 | 940 | 950 | 960 |
| TAMPPLTLGI | FERSCRKENM | LKYPELYKTS | QNALDFNTKV | FWVHCLNGLF | HSVILFWFPL |
| 970 | 980 | 990 | 1000 | 1010 | 1020 |
| KALQYGTVFG | NGKTSDYLLL | GNFVYTFVVI | TVCLKAGLET | SYWTWFSHIA | IWGSIALWVV |
| 1030 | 1040 | 1050 | 1060 | 1070 | 1080 |
| FFGIYSSLWP | AVPMAPDMSG | EAAMLFSSGV | FWVGLLSIPV | ASLLLDVLYK | VIKRTAFKTL |
| 1090 | 1100 | 1110 | 1120 | 1130 | 1140 |
| VDEVQELEAK | SQDPGAVVLG | KSLTERAQLL | KNVFKKNHVN | LYRSESLQQN | LLHGYAFSQD |
| 1150 | 1160 | ||||
| ENGIVSQSEV | IRAYDTTKQR | PDEW |