P52952
Gene name |
NKX2-5 (CSX, NKX2.5, NKX2E) |
Protein name |
Homeobox protein Nkx-2.5 |
Names |
Cardiac-specific homeobox, Homeobox protein CSX, Homeobox protein NK-2 homolog E |
Species |
Homo sapiens (Human) |
KEGG Pathway |
hsa:1482 |
EC number |
|
Protein Class |
|
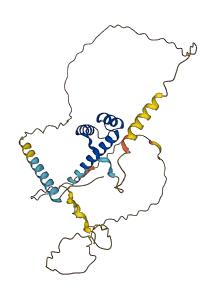
Descriptions
The autoinhibited protein was predicted that may have potential autoinhibitory elements via cis-regPred.
Autoinhibitory domains (AIDs)
Target domain |
|
Relief mechanism |
|
Assay |
cis-regPred |
Accessory elements
No accessory elements
Autoinhibited structure

Activated structure

5 structures for P52952
| Entry ID | Method | Resolution | Chain | Position | Source |
|---|---|---|---|---|---|
| 3RKQ | X-ray | 170 A | A/B | 138-192 | PDB |
| 4S0H | X-ray | 282 A | B/F | 142-192 | PDB |
| 6WC2 | X-ray | 210 A | M/N/O | 137-197 | PDB |
| 6WC5 | X-ray | 290 A | I/N | 140-196 | PDB |
| AF-P52952-F1 | Predicted | AlphaFoldDB |
421 variants for P52952
| Variant ID(s) | Position | Change | Description | Diseaes Association | Provenance |
|---|---|---|---|---|---|
|
RCV000702042 RCV002388316 rs769233111 CA3563864 |
5 | P>S | Atrial septal defect 7 [ClinVar] | Yes |
ClinGen ClinVar ExAC TOPMed dbSNP gnomAD |
| VAR_038212 | 7 | L>P | ASD7; somatic mutation [UniProt] | Yes | UniProt |
|
RCV000023020 rs387906773 CA212677 VAR_038213 |
15 | K>I | Atrial septal defect 7 ASD7 [ClinVar, UniProt] | Yes |
ClinGen ClinVar UniProt Ensembl dbSNP |
| VAR_038214 | 19 | N>S | ASD7; somatic mutation [UniProt] | Yes | UniProt |
|
RCV000171013 CA214394 RCV000618034 RCV000987633 RCV000030618 RCV000009574 VAR_038215 rs104893904 RCV000514277 |
21 | E>Q | Tetralogy of Fallot Tetralogy of fallot (tof) Atrial septal defect 7 Congenital heart disease TOF and ASD7 [ClinVar, Ensembl, UniProt] | Yes |
ClinGen ClinVar UniProt ESP ExAC TOPMed dbSNP gnomAD |
|
CA132260566 RCV002377106 RCV002483450 RCV000786392 RCV000542359 VAR_038216 rs201442000 |
22 | Q>P | Atrial septal defect 7 ASD7 and TOF [ClinVar, UniProt] | Yes |
ClinGen ClinVar UniProt ExAC TOPMed dbSNP gnomAD |
|
CA206627 RCV000727462 RCV000193266 RCV002298499 RCV000618509 RCV000477570 rs201442000 |
22 | Q>R | Atrial septal defect 7 [ClinVar] | Yes |
ClinGen ClinVar ExAC TOPMed dbSNP gnomAD |
|
RCV000030339 rs28936670 RCV000023019 VAR_010116 CA120055 RCV000037968 RCV000009572 RCV000619696 RCV000023017 RCV000023018 RCV000987632 RCV000009573 |
25 | R>C | Hypothyroidism, congenital, nongoitrous, 5 Tetralogy of Fallot Tetralogy of fallot (tof) Atrial septal defect 7 Hypoplastic left heart syndrome 2 (hlhs2) Congenital heart disease Hypothyroidism, congenital, nongoitrous, 5 (chng5) Hypoplastic left heart syndrome 2 Aortic arch interruption ASD7, TOF, CHNG5, HLHS2 and CTHM; unknown pathological significance; exhibits significant functional impairment with reduction of transactivation properties and dominant-negative effect; the mutant protein activity on the DIO2, TG and TPO promoters is significantly impaired [ClinVar, Ensembl, UniProt] | Yes |
ClinGen ClinVar UniProt 1000Genomes ESP ExAC TOPMed dbSNP gnomAD |
|
RCV003165900 CA362163723 rs1279595214 RCV000703980 |
28 | A>T | Atrial septal defect 7 [ClinVar] | Yes |
ClinGen ClinVar TOPMed dbSNP gnomAD |
|
rs1425683417 RCV000820710 CA362163708 |
30 | A>D | Atrial septal defect 7 [ClinVar] | Yes |
ClinGen ClinVar dbSNP gnomAD |
|
CA362163711 rs1304644050 RCV001858880 |
30 | A>T | Atrial septal defect 7 [ClinVar] | Yes |
ClinGen ClinVar dbSNP gnomAD |
|
RCV001208962 rs1360215151 CA362163705 |
31 | G>R | Atrial septal defect 7 [ClinVar] | Yes |
ClinGen ClinVar TOPMed dbSNP gnomAD |
|
RCV001061180 RCV002489668 CA3563847 rs776310516 |
32 | E>D | Atrial septal defect 7 [ClinVar] | Yes |
ClinGen ClinVar ExAC TOPMed dbSNP gnomAD |
|
RCV001203285 rs1234717083 |
39 | A>G | Atrial septal defect 7 [ClinVar] | Yes |
ClinVar dbSNP |
|
RCV001573768 rs113818864 RCV002408739 CA302112 RCV000470357 |
42 | A>P | Atrial septal defect 7 [ClinVar] | Yes |
ClinGen ClinVar 1000Genomes ESP ExAC TOPMed dbSNP gnomAD |
|
VAR_038217 rs779548360 CA132260457 |
45 | S>P | ASD7; somatic mutation [UniProt] | Yes |
ClinGen UniProt ExAC TOPMed dbSNP gnomAD |
|
rs1561621507 RCV000690794 |
50 | A>missing | Atrial septal defect 7 [ClinVar] | Yes |
ClinVar dbSNP |
|
rs753937287 CA3563841 VAR_038218 |
51 | F>L | ASD7; somatic mutation [UniProt] | Yes |
ClinGen UniProt ExAC dbSNP gnomAD |
|
RCV001216063 rs1761438700 |
54 | E>missing | Atrial septal defect 7 [ClinVar] | Yes |
ClinVar dbSNP |
|
rs549161381 RCV002476505 RCV001321197 RCV001773641 CA3563838 |
57 | A>S | Atrial septal defect 7 [ClinVar] | Yes |
ClinGen ClinVar ExAC dbSNP gnomAD |
|
rs1012750146 RCV000621821 CA132260438 RCV001038484 |
58 | G>V | Atrial septal defect 7 [ClinVar] | Yes |
ClinGen ClinVar TOPMed dbSNP gnomAD |
|
rs387906775 VAR_067586 RCV000023024 CA212680 |
59 | P>A | Ventricular septal defect 3 Ventricular septal defect 3 (vsd3) VSD3; significantly reduced activation of NPPA gene compared to wild-type [ClinVar, Ensembl, UniProt] | Yes |
ClinGen ClinVar UniProt Ensembl dbSNP |
|
RCV001595076 rs766199339 RCV002493668 RCV002412026 RCV001318738 CA3563837 |
60 | E>Q | Atrial septal defect 7 [ClinVar] | Yes |
ClinGen ClinVar ExAC TOPMed dbSNP gnomAD |
|
RCV000203543 CA279938 rs864321650 |
61 | A>G | Congenital heart disease [ClinVar] | Yes |
ClinGen ClinVar TOPMed dbSNP gnomAD |
|
VAR_038219 RCV002409458 RCV002255174 RCV002481996 rs530270916 RCV001055047 CA3563832 |
63 | A>V | Atrial septal defect 7 ASD7 [ClinVar, UniProt] | Yes |
ClinGen ClinVar UniProt 1000Genomes ExAC TOPMed dbSNP gnomAD |
| VAR_038220 | 69 | L>P | ASD7; somatic mutation [UniProt] | Yes | UniProt |
|
RCV000644445 rs1032793565 CA132260410 |
69 | L>R | Atrial septal defect 7 [ClinVar] | Yes |
ClinGen ClinVar TOPMed dbSNP |
|
RCV000009577 rs606231358 |
72 | E>missing | Atrial septal defect 7 [ClinVar] | Yes |
ClinVar dbSNP |
|
rs606231359 RCV000009578 |
77 | P>missing | Atrial septal defect 7 [ClinVar] | Yes |
ClinVar dbSNP |
| VAR_038221 | 77 | P>L | ASD7; somatic mutation [UniProt] | Yes | UniProt |
|
RCV000644447 rs904474688 CA132260367 |
81 | K>E | Atrial septal defect 7 [ClinVar] | Yes |
ClinGen ClinVar TOPMed dbSNP |
|
RCV001222867 rs1761434175 |
83 | A>missing | Atrial septal defect 7 [ClinVar] | Yes |
ClinVar dbSNP |
|
RCV002476516 CA362163399 rs1182777346 RCV002431920 RCV001322934 |
83 | A>G | Atrial septal defect 7 [ClinVar] | Yes |
ClinGen ClinVar dbSNP gnomAD |
|
RCV000009581 rs606231360 |
88 | A>missing | Atrial septal defect 7 [ClinVar] | Yes |
ClinVar dbSNP |
|
rs1761432750 RCV001344340 |
89 | A>P | Atrial septal defect 7 [ClinVar] | Yes |
ClinVar dbSNP |
|
CA132260347 RCV000549035 rs1009994744 |
94 | P>S | Atrial septal defect 7 [ClinVar] | Yes |
ClinGen ClinVar TOPMed dbSNP |
|
CA3563814 RCV001302062 rs763729448 RCV002437025 |
95 | R>L | Atrial septal defect 7 [ClinVar] | Yes |
ClinGen ClinVar ExAC dbSNP gnomAD |
|
CA3563813 rs760088847 RCV000798171 |
96 | A>V | Atrial septal defect 7 [ClinVar] | Yes |
ClinGen ClinVar ExAC dbSNP gnomAD |
|
CA3563812 RCV000226256 rs550046293 |
100 | P>A | Atrial septal defect 7 [ClinVar] | Yes |
ClinGen ClinVar ExAC TOPMed dbSNP gnomAD |
|
RCV002440511 CA132260336 RCV000701268 rs550046293 |
100 | P>T | Atrial septal defect 7 [ClinVar] | Yes |
ClinGen ClinVar ExAC TOPMed dbSNP gnomAD |
|
RCV001058874 rs1761430125 |
104 | K>* | Atrial septal defect 7 [ClinVar] | Yes |
ClinVar dbSNP |
|
CA362163209 RCV000794200 rs1581111049 |
108 | A>V | Atrial septal defect 7 [ClinVar] | Yes |
ClinGen ClinVar Ensembl dbSNP |
| VAR_038222 | 114 | C>R | ASD7; somatic mutation [UniProt] | Yes | UniProt |
| VAR_038223 | 114 | C>S | ASD7; somatic mutation [UniProt] | Yes | UniProt |
|
RCV003129941 RCV000617599 RCV002528814 CA362162051 rs760723447 |
114 | C>W | Atrial septal defect 7 [ClinVar] | Yes |
ClinGen ClinVar ExAC dbSNP gnomAD |
| VAR_038224 | 118 | K>R | ASD7; somatic mutation [UniProt] | Yes | UniProt |
|
RCV002450952 CA3563717 RCV000414455 RCV001321327 RCV002223836 RCV002488852 rs369025518 |
119 | A>E | Atrial septal defect 7 [ClinVar] | Yes |
ClinGen ClinVar ESP ExAC TOPMed dbSNP gnomAD |
|
RCV000171007 CA120058 RCV000009584 RCV000620259 RCV001529235 VAR_047869 rs137852684 RCV000230156 |
119 | A>S | Hypothyroidism, congenital, nongoitrous, 5 Atrial septal defect 7 Hypothyroidism, congenital, nongoitrous, 5 (chng5) CHNG5; exhibits a significant functional impairment with reduction of transactivation properties and dominant-negative effect which was associated with reduced DNA binding [ClinVar, Ensembl, UniProt] | Yes |
ClinGen ClinVar UniProt 1000Genomes ESP ExAC TOPMed dbSNP gnomAD |
| VAR_038225 | 124 | K>R | ASD7; somatic mutation [UniProt] | Yes | UniProt |
|
rs1561619801 RCV000707466 |
126 | E>missing | Atrial septal defect 7 [ClinVar] | Yes |
ClinVar dbSNP |
|
rs1320947604 CA362161979 RCV000786393 RCV000644448 |
126 | E>A | Atrial septal defect 7 [ClinVar] | Yes |
ClinGen ClinVar dbSNP gnomAD |
| VAR_038226 | 126 | E>V | ASD7; somatic mutation [UniProt] | Yes | UniProt |
|
rs387906774 RCV002509168 VAR_038227 CA212678 RCV000023021 |
127 | A>E | Atrial septal defect 7 ASD7 [ClinVar, UniProt] | Yes |
ClinGen ClinVar UniProt TOPMed dbSNP gnomAD |
|
RCV001208971 CA362161960 rs750029908 RCV002491634 |
129 | N>K | Atrial septal defect 7 [ClinVar] | Yes |
ClinGen ClinVar ExAC TOPMed dbSNP gnomAD |
|
CA279909 rs864321648 RCV000203502 |
131 | E>K | Congenital heart disease [ClinVar] | Yes |
ClinGen ClinVar Ensembl dbSNP |
|
RCV000617877 RCV000808237 CA3563706 rs754394393 |
132 | R>P | Atrial septal defect 7 [ClinVar] | Yes |
ClinGen ClinVar ExAC TOPMed dbSNP gnomAD |
|
CA362161940 rs1184594159 VAR_038228 |
133 | P>S | ASD7; somatic mutation [UniProt] | Yes |
ClinGen UniProt dbSNP gnomAD |
| VAR_038229 | 135 | A>T | ASD7; somatic mutation [UniProt] | Yes | UniProt |
| VAR_038230 | 142 | R>C | ASD7 [UniProt] | Yes | UniProt |
|
RCV000644450 rs1431464297 CA362161880 |
144 | L>F | Atrial septal defect 7 [ClinVar] | Yes |
ClinGen ClinVar dbSNP gnomAD |
|
VAR_038231 RCV001212171 rs747932354 |
144 | L>P | Atrial septal defect 7 ASD7; somatic mutation [ClinVar, UniProt] | Yes |
ClinVar dbSNP UniProt |
|
rs1761360431 RCV001261993 |
147 | Q>missing | Tetralogy of Fallot [ClinVar] | Yes |
ClinVar dbSNP |
|
CA279927 RCV000203525 rs864321649 |
148 | A>E | Congenital heart disease [ClinVar] | Yes |
ClinGen ClinVar dbSNP gnomAD |
|
RCV000549940 CA362161847 rs1230869762 |
149 | Q>H | Atrial septal defect 7 [ClinVar] | Yes |
ClinGen ClinVar dbSNP gnomAD |
|
RCV000622055 RCV001227312 rs201582515 RCV002223229 CA132259297 RCV002491307 |
150 | V>I | Atrial septal defect 7 [ClinVar] | Yes |
ClinGen ClinVar 1000Genomes TOPMed dbSNP |
|
CA212702 RCV000144176 rs587782928 |
154 | E>G | Atrial septal defect 7 [ClinVar] | Yes |
ClinGen ClinVar Ensembl dbSNP |
|
rs587784066 RCV000146752 |
157 | F>missing | Malformation of the heart and great vessels [ClinVar] | Yes |
ClinVar dbSNP |
|
RCV000009585 rs137852685 VAR_047870 CA120059 |
161 | R>P | Hypothyroidism, congenital, nongoitrous, 5 Hypothyroidism, congenital, nongoitrous, 5 (chng5) CHNG5; exhibits a significant functional impairment with reduction of transactivation properties and dominant-negative effect which was associated with reduced DNA binding [ClinVar, Ensembl, UniProt] | Yes |
ClinGen ClinVar UniProt 1000Genomes ExAC dbSNP gnomAD |
|
rs1456289029 RCV001052873 |
162 | Y>* | Atrial septal defect 7 [ClinVar] | Yes |
ClinVar dbSNP |
|
rs1761357696 RCV001035517 |
167 | E>K | Atrial septal defect 7 [ClinVar] | Yes |
ClinVar dbSNP |
|
RCV000009569 CA212654 rs104893901 |
170 | Q>* | Atrial septal defect 7 [ClinVar] | Yes |
ClinGen ClinVar Ensembl dbSNP |
|
CA10582413 RCV000232942 rs878854704 |
172 | A>T | Atrial septal defect 7 [ClinVar] | Yes |
ClinGen ClinVar Ensembl dbSNP |
|
rs1761356979 RCV001320731 RCV002499622 |
174 | V>G | Atrial septal defect 7 [ClinVar] | Yes |
ClinVar dbSNP |
|
RCV002504322 RCV001235147 rs1761356915 |
175 | L>P | Atrial septal defect 7 [ClinVar] | Yes |
ClinVar dbSNP |
|
VAR_003752 rs104893900 CA212653 RCV000009568 |
178 | T>M | Atrial septal defect 7 ASD7 [ClinVar, UniProt] | Yes |
ClinGen ClinVar UniProt Ensembl dbSNP |
|
RCV000588165 CA214180 rs72554028 |
181 | Q>H | Atrial septal defect 7 [ClinVar] | Yes |
ClinGen ClinVar 1000Genomes ESP ExAC TOPMed dbSNP gnomAD |
|
RCV000009586 CA120060 VAR_038232 rs137852686 |
183 | K>E | Atrioventricular septal defect, somatic ASD7; somatic mutation [ClinVar, UniProt] | Yes |
ClinGen ClinVar UniProt Ensembl dbSNP |
|
RCV001344953 rs1761355968 |
185 | W>R | Atrial septal defect 7 [ClinVar] | Yes |
ClinVar dbSNP |
| VAR_038233 | 187 | Q>H | ASD7 [UniProt] | Yes | UniProt |
| VAR_010117 | 188 | N>K | ASD7 [UniProt] | Yes | UniProt |
| VAR_010118 | 189 | R>G | ASD7 [UniProt] | Yes | UniProt |
|
RCV000702426 rs786205824 RCV000171010 CA302106 |
189 | R>P | Atrial septal defect 7 [ClinVar] | Yes |
ClinGen ClinVar dbSNP gnomAD |
|
CA362161594 rs786205824 RCV000700821 |
189 | R>Q | Atrial septal defect 7 [ClinVar] | Yes |
ClinGen ClinVar dbSNP gnomAD |
|
CA212658 rs104893906 VAR_038234 RCV000009582 |
190 | R>C | Atrial septal defect 7 ASD7 [ClinVar, UniProt] | Yes |
ClinGen ClinVar UniProt TOPMed dbSNP |
| VAR_010119 | 191 | Y>C | ASD7 [UniProt] | Yes | UniProt |
| VAR_038235 | 192 | K>R | ASD7; somatic mutation [UniProt] | Yes | UniProt |
| VAR_038236 | 192 | K>T | ASD7; somatic mutation [UniProt] | Yes | UniProt |
| VAR_038237 | 194 | K>R | ASD7; somatic mutation [UniProt] | Yes | UniProt |
|
RCV001852050 RCV002354420 rs774482632 RCV000171011 CA302108 RCV002492707 |
197 | R>P | Atrial septal defect 7 [ClinVar] | Yes |
ClinGen ClinVar ExAC TOPMed dbSNP |
|
RCV000009570 CA212656 rs104893903 |
198 | Q>* | Atrial septal defect 7 [ClinVar] | Yes |
ClinGen ClinVar Ensembl dbSNP |
|
RCV000786390 RCV001265720 rs1554093461 RCV000644449 |
202 | L>missing | Atrial septal defect 7 Inborn genetic diseases [ClinVar] | Yes |
ClinVar dbSNP |
|
rs768499098 RCV002224022 CA3563682 RCV001208765 |
202 | L>P | Atrial septal defect 7 [ClinVar] | Yes |
ClinGen ClinVar ExAC TOPMed dbSNP gnomAD |
|
CA3563680 rs771533553 RCV000621713 RCV001240727 |
203 | E>G | Atrial septal defect 7 [ClinVar] | Yes |
ClinGen ClinVar ExAC TOPMed dbSNP gnomAD |
| VAR_038238 | 205 | V>E | ASD7; somatic mutation [UniProt] | Yes | UniProt |
|
RCV000144177 rs587782929 |
207 | L>missing | Atrial septal defect 7 [ClinVar] | Yes |
ClinVar dbSNP |
|
CA302110 RCV001704244 rs3729754 RCV000470087 RCV002354421 |
211 | P>L | Atrial septal defect 7 [ClinVar] | Yes |
ClinGen ClinVar 1000Genomes ExAC TOPMed dbSNP gnomAD |
|
RCV001305735 rs1761352648 |
212 | P>missing | Atrial septal defect 7 [ClinVar] | Yes |
ClinVar dbSNP |
|
rs372282873 RCV001068644 CA3563677 RCV001593249 RCV002482121 |
212 | P>R | Atrial septal defect 7 [ClinVar] | Yes |
ClinGen ClinVar ESP ExAC TOPMed dbSNP gnomAD |
|
rs1250491634 RCV001052178 CA362161454 |
213 | P>A | Atrial septal defect 7 [ClinVar] | Yes |
ClinGen ClinVar TOPMed dbSNP gnomAD |
|
RCV000462970 RCV000617268 RCV000786394 rs746833511 |
214 | P>missing | Atrial septal defect 7 [ClinVar] | Yes |
ClinVar dbSNP |
|
RCV000456338 RCV001293212 RCV002367610 rs746833511 |
214 | P>missing | Atrial septal defect 7 Primary dilated cardiomyopathy [ClinVar] | Yes |
ClinVar dbSNP |
|
RCV002482846 rs104893905 VAR_038239 CA248606 RCV001588805 RCV000009575 |
216 | R>C | Tetralogy of Fallot Tetralogy of fallot (tof) Atrial septal defect 7 TOF and ASD7 [ClinVar, Ensembl, UniProt] | Yes |
ClinGen ClinVar UniProt dbSNP gnomAD |
|
CA3563669 rs751684900 RCV002504484 RCV001315155 |
217 | R>K | Atrial septal defect 7 [ClinVar] | Yes |
ClinGen ClinVar ExAC TOPMed dbSNP gnomAD |
|
RCV002360590 RCV000644451 CA3563667 rs760305842 |
219 | A>T | Atrial septal defect 7 [ClinVar] | Yes |
ClinGen ClinVar ExAC dbSNP gnomAD |
|
RCV000525369 rs104893902 RCV002482847 VAR_038240 RCV000009576 RCV002362574 CA248607 |
219 | A>V | Tetralogy of Fallot Tetralogy of fallot (tof) Atrial septal defect 7 ASD7 and TOF; somatic mutation [ClinVar, Ensembl, UniProt] | Yes |
ClinGen ClinVar UniProt TOPMed dbSNP |
|
RCV002530931 rs1554093444 RCV000587249 CA362161407 |
221 | P>L | Atrial septal defect 7 [ClinVar] | Yes |
ClinGen ClinVar Ensembl dbSNP |
|
RCV000818694 rs1581108237 |
223 | L>missing | Atrial septal defect 7 [ClinVar] | Yes |
ClinVar dbSNP |
|
rs866974584 CA132259062 RCV001210640 RCV002365948 |
225 | R>C | Atrial septal defect 7 [ClinVar] | Yes |
ClinGen ClinVar TOPMed dbSNP gnomAD |
|
RCV001064439 VAR_038241 CA3563664 rs760528062 |
226 | D>N | Atrial septal defect 7 ASD7; somatic mutation [ClinVar, UniProt] | Yes |
ClinGen ClinVar UniProt ExAC dbSNP gnomAD |
|
RCV001577986 rs397515399 VAR_069590 RCV000032628 CA130288 |
236 | P>H | Familial isolated congenital asplenia found in patients with isolated congenital asplenia; unknown pathological significance; does not affect DNA binding; impairs transactivation activity [ClinVar, UniProt] | Yes |
ClinGen ClinVar UniProt ExAC TOPMed dbSNP gnomAD |
|
CA362161325 RCV001210751 rs770192204 |
236 | P>S | Atrial septal defect 7 [ClinVar] | Yes |
ClinGen ClinVar ExAC TOPMed dbSNP gnomAD |
|
CA3563656 RCV002526435 RCV000471990 rs770192204 |
236 | P>T | Atrial septal defect 7 Inborn genetic diseases [ClinVar] | Yes |
ClinGen ClinVar ExAC TOPMed dbSNP gnomAD |
|
rs1554093433 RCV000626863 RCV001049571 CA362161316 |
237 | Y>* | Atrial septal defect 7 Primary dilated cardiomyopathy [ClinVar] | Yes |
ClinGen ClinVar Ensembl dbSNP |
|
rs1761349696 RCV001065033 RCV003160536 |
237 | Y>H | Atrial septal defect 7 [ClinVar] | Yes |
ClinVar dbSNP |
|
RCV000144178 rs587782930 |
241 | Y>missing | Atrial septal defect 7 [ClinVar] | Yes |
ClinVar dbSNP |
|
CA362161288 RCV000644444 rs867226708 |
242 | G>R | Atrial septal defect 7 [ClinVar] | Yes |
ClinGen ClinVar Ensembl dbSNP |
|
rs867226708 CA132259021 RCV001049998 |
242 | G>S | Atrial septal defect 7 [ClinVar] | Yes |
ClinGen ClinVar Ensembl dbSNP |
|
rs1131691343 RCV000494374 CA362161252 RCV001851351 |
248 | Y>C | Atrial septal defect 7 [ClinVar] | Yes |
ClinGen ClinVar dbSNP gnomAD |
| VAR_038242 | 248 | Y>H | ASD7; somatic mutation [UniProt] | Yes | UniProt |
|
RCV000234875 rs879253754 |
251 | N>missing | Atrial septal defect 7 [ClinVar] | Yes |
ClinVar dbSNP |
|
rs765528024 RCV001056527 RCV000620204 RCV002483718 CA3563649 COSM737687 |
251 | N>K | lung Atrial septal defect 7 [Cosmic, ClinVar] | Yes |
ClinGen cosmic curated ClinVar ExAC TOPMed dbSNP gnomAD |
|
RCV001343024 rs1327849028 RCV001762576 CA362161232 |
251 | N>S | Atrial septal defect 7 Variant assessed as Somatic; 0.0 impact. [ClinVar, NCI-TCGA] | Yes |
ClinGen ClinVar NCI-TCGA dbSNP gnomAD |
|
RCV001327140 rs762090105 RCV001593059 CA3563648 RCV000852563 RCV002390732 |
252 | A>V | Atrial septal defect 7 Hypertrophic cardiomyopathy [ClinVar] | Yes |
ClinGen ClinVar ExAC TOPMed dbSNP gnomAD |
|
RCV000009583 rs104893907 RCV000240621 CA212659 CA10586352 |
256 | Y>* | Atrial septal defect 7 [ClinVar] | Yes |
ClinGen ClinVar Ensembl dbSNP |
|
rs387906776 CA212681 RCV000023025 |
257 | P>A | Ventricular septal defect 3 Ventricular septal defect 3 (vsd3) [ClinVar, Ensembl] | Yes |
ClinGen ClinVar ExAC TOPMed dbSNP gnomAD |
|
RCV001061626 rs387906776 RCV000294434 RCV002503988 RCV003165731 CA10604577 |
257 | P>T | Atrial septal defect 7 Ventricular septal defect 3 (vsd3) [ClinVar, Ensembl] | Yes |
ClinGen ClinVar ExAC TOPMed dbSNP gnomAD |
|
RCV000146756 RCV000550275 rs587784067 RCV001582613 RCV002483283 |
262 | A>missing | Atrial septal defect 7 Malformation of the heart and great vessels [ClinVar] | Yes |
ClinVar dbSNP |
|
CA3563636 RCV001202387 rs780688010 |
267 | G>S | Atrial septal defect 7 [ClinVar] | Yes |
ClinGen ClinVar ExAC TOPMed dbSNP gnomAD |
|
RCV001313496 rs1761346424 |
268 | Y>* | Atrial septal defect 7 [ClinVar] | Yes |
ClinVar dbSNP |
|
CA170785 RCV000617639 RCV000144179 RCV000644453 rs587782931 |
270 | C>Y | Atrial septal defect 7 [ClinVar] | Yes |
ClinGen ClinVar ExAC TOPMed dbSNP gnomAD |
|
RCV000477547 rs1060503097 CA16612032 RCV000660565 |
275 | P>L | Atrial septal defect 7 [ClinVar] | Yes |
ClinGen ClinVar dbSNP gnomAD |
|
RCV000704559 RCV002477624 VAR_038243 RCV002424703 CA3563630 RCV001470939 rs368366482 |
275 | P>T | Atrial septal defect 7 ASD7 [ClinVar, UniProt] | Yes |
ClinGen ClinVar UniProt 1000Genomes ESP ExAC TOPMed dbSNP gnomAD |
|
RCV002429460 CA3563628 RCV003224281 RCV000434919 RCV000644446 rs751564052 |
276 | A>G | Atrial septal defect 7 [ClinVar] | Yes |
ClinGen ClinVar ExAC TOPMed dbSNP gnomAD |
|
rs1223599871 VAR_038244 CA362161073 |
279 | S>F | ASD7; somatic mutation [UniProt] | Yes |
ClinGen UniProt dbSNP gnomAD |
| VAR_038245 | 279 | S>P | ASD7; somatic mutation [UniProt] | Yes | UniProt |
|
RCV000620094 rs761596254 RCV000526273 CA3563623 |
280 | P>L | Atrial septal defect 7 [ClinVar] | Yes |
ClinGen ClinVar ExAC TOPMed dbSNP gnomAD |
|
CA362161062 RCV002489557 rs1206339157 RCV002409381 RCV001038821 |
281 | A>E | Atrial septal defect 7 [ClinVar] | Yes |
ClinGen ClinVar TOPMed dbSNP gnomAD |
| VAR_038246 | 281 | A>V | ASD7; somatic mutation [UniProt] | Yes | UniProt |
|
RCV000421219 rs375086983 VAR_067587 RCV002490404 RCV000539285 RCV002408478 RCV000023023 CA212679 |
283 | P>Q | Atrial septal defect 7 Ventricular septal defect 3 Ventricular septal defect 3 (vsd3) VSD3 [ClinVar, Ensembl, UniProt] | Yes |
ClinGen ClinVar UniProt ExAC TOPMed dbSNP gnomAD |
|
rs1761344289 RCV001037090 |
284 | A>V | Atrial septal defect 7 [ClinVar] | Yes |
ClinVar dbSNP |
| VAR_038247 | 286 | A>V | ASD7; somatic mutation [UniProt] | Yes | UniProt |
|
CA132258798 RCV002370079 RCV000794908 rs936204422 RCV001772050 |
290 | N>S | Atrial septal defect 7 [ClinVar] | Yes |
ClinGen ClinVar Ensembl dbSNP |
|
VAR_067588 rs756974215 RCV002374804 RCV000462724 RCV002489071 RCV000023022 |
291 | N>missing | Atrial septal defect 7 CTMH Double outlet right ventricle [ClinVar, UniProt] | Yes |
ClinVar UniProt dbSNP |
| VAR_038248 | 294 | N>H | ASD7; somatic mutation [UniProt] | Yes | UniProt |
|
CA3563614 RCV001066168 rs150581386 RCV002374974 RCV001352385 CA3563612 RCV002482097 |
295 | F>L | Atrial septal defect 7 [ClinVar] | Yes |
ClinGen ClinVar ESP ExAC TOPMed dbSNP gnomAD |
|
RCV000519820 CA362160957 RCV002525255 rs1358735679 |
296 | G>S | Atrial septal defect 7 [ClinVar] | Yes |
ClinGen ClinVar TOPMed dbSNP |
|
CA3563610 COSM1259157 RCV000532333 rs569535312 RCV000617437 |
297 | V>F | Atrial septal defect 7 oesophagus [ClinVar, Cosmic] | Yes |
ClinGen cosmic curated ClinVar 1000Genomes ExAC dbSNP gnomAD |
|
RCV000623364 RCV001193907 rs778545351 RCV001038060 RCV002483744 |
298 | G>missing | Atrial septal defect 7 Inborn genetic diseases [ClinVar] | Yes |
ClinVar dbSNP |
|
rs137852683 VAR_038249 RCV000009579 RCV000009580 CA120057 |
299 | D>G | Atrial septal defect 7 Atrioventricular septal defect, somatic ASD7; somatic mutation [ClinVar, UniProt] | Yes |
ClinGen ClinVar UniProt Ensembl dbSNP |
|
CA3563601 rs772495396 RCV001762579 RCV001343963 |
303 | V>F | Atrial septal defect 7 [ClinVar] | Yes |
ClinGen ClinVar ExAC TOPMed dbSNP gnomAD |
| VAR_038250 | 305 | S>G | ASD7; somatic mutation [UniProt] | Yes | UniProt |
|
CA3563590 rs201249977 RCV001727746 RCV000998493 RCV000540219 |
315 | V>L | Atrial septal defect 7 [ClinVar] | Yes |
ClinGen ClinVar 1000Genomes ESP ExAC TOPMed dbSNP gnomAD |
|
rs1581107775 RCV000824256 CA915942749 |
318 | L>A | Atrial septal defect 7 [ClinVar] | Yes |
ClinGen ClinVar Ensembl dbSNP |
| VAR_038251 | 320 | G>S | ASD7; somatic mutation [UniProt] | Yes | UniProt |
| VAR_038252 | 322 | R>Q | ASD7; somatic mutation [UniProt] | Yes | UniProt |
| VAR_038253 | 323 | A>T | ASD7 and TOF [UniProt] | Yes | UniProt |
|
CA362163872 rs1270909226 |
4 | S>G | No |
ClinGen TOPMed |
|
|
rs1341734909 CA362163868 |
4 | S>N | No |
ClinGen TOPMed |
|
|
CA362163856 rs1561621781 |
6 | A>G | No |
ClinGen Ensembl |
|
|
rs780812253 CA3563862 |
6 | A>T | No |
ClinGen ExAC gnomAD |
|
|
CA362163854 rs1277858223 |
7 | L>V | No |
ClinGen TOPMed |
|
|
rs1485780506 CA362163848 |
8 | T>A | No |
ClinGen gnomAD |
|
|
rs768954078 CA3563861 |
8 | T>M | No |
ClinGen ExAC gnomAD |
|
|
RCV000489523 CA362163821 rs1085307815 |
12 | F>S | No |
ClinGen ClinVar Ensembl dbSNP |
|
|
CA362163823 rs1363368305 |
12 | F>V | No |
ClinGen gnomAD |
|
|
rs17052019 VAR_049581 CA132260599 |
16 | D>A | No |
ClinGen UniProt Ensembl dbSNP |
|
|
rs750904275 CA3563857 |
16 | D>N | No |
ClinGen ExAC gnomAD |
|
|
CA362163790 rs1176053334 |
17 | I>V | No |
ClinGen TOPMed gnomAD |
|
|
rs1471444031 RCV000621382 CA362163769 |
20 | L>P | No |
ClinGen ClinVar dbSNP gnomAD |
|
|
rs2277923 CA362163762 CA362163761 |
21 | E>D | No |
ClinGen 1000Genomes ESP ExAC TOPMed gnomAD |
|
|
CA3563855 rs764389026 |
22 | Q>E | No |
ClinGen ExAC TOPMed gnomAD |
|
|
CA362163759 rs764389026 |
22 | Q>K | No |
ClinGen ExAC TOPMed gnomAD |
|
|
CA3563854 rs751398261 |
24 | Q>R | No |
ClinGen ExAC gnomAD |
|
|
rs762957966 CA3563853 |
25 | R>L | No |
ClinGen ExAC gnomAD |
|
|
CA362163733 rs1375476320 |
26 | S>N | No |
ClinGen gnomAD |
|
|
CA362163717 rs1554093718 RCV000617626 |
29 | A>T | No |
ClinGen ClinVar Ensembl dbSNP |
|
|
CA3563849 rs769355297 |
31 | G>V | No |
ClinGen ExAC gnomAD |
|
|
rs552617433 CA3563848 |
32 | E>K | No |
ClinGen 1000Genomes ExAC |
|
|
CA132260474 rs1048146799 |
33 | L>F | No |
ClinGen Ensembl |
|
|
CA3563846 rs768386936 |
35 | A>V | No |
ClinGen ExAC |
|
|
rs1172036454 CA362163676 |
36 | R>C | No |
ClinGen gnomAD |
|
|
rs948440843 CA132260470 |
36 | R>L | No |
ClinGen Ensembl |
|
|
CA362163668 rs1302984436 |
38 | E>K | No |
ClinGen TOPMed |
|
|
CA362163657 rs1234717083 |
39 | A>E | No |
ClinGen TOPMed |
|
| TCGA novel | 40 | T>I | Variant assessed as Somatic; impact. [NCI-TCGA] | No | NCI-TCGA |
|
rs113818864 CA362163642 |
42 | A>T | No |
ClinGen 1000Genomes ESP ExAC TOPMed gnomAD |
|
|
CA3563843 rs779548360 |
45 | S>A | No |
ClinGen ExAC TOPMed gnomAD |
|
| TCGA novel | 45 | S>F | Variant assessed as Somatic; impact. [NCI-TCGA] | No | NCI-TCGA |
|
CA362163603 rs1284932088 |
48 | L>P | No |
ClinGen gnomAD |
|
|
rs777670902 CA3563840 |
52 | K>Q | No |
ClinGen ExAC gnomAD |
|
|
rs867314114 CA132260451 |
54 | E>D | No |
ClinGen TOPMed |
|
|
CA362163563 rs1261741680 |
54 | E>V | No |
ClinGen TOPMed |
|
|
CA132260444 rs567939950 |
55 | A>T | No |
ClinGen Ensembl |
|
|
rs1360861650 CA362163552 |
56 | Y>C | No |
ClinGen gnomAD |
|
|
rs1346280352 CA362163547 |
57 | A>D | No |
ClinGen gnomAD |
|
|
CA132260439 rs549161381 |
57 | A>P | No |
ClinGen ExAC gnomAD |
|
|
CA362163534 rs766199339 |
60 | E>K | No |
ClinGen ExAC TOPMed gnomAD |
|
|
CA362163523 rs864321650 |
61 | A>V | No |
ClinGen TOPMed gnomAD |
|
|
rs530270916 CA3563833 |
63 | A>E | No |
ClinGen 1000Genomes ExAC TOPMed gnomAD |
|
|
rs765129454 CA3563834 |
63 | A>T | No |
ClinGen ExAC gnomAD |
|
|
CA3563831 rs763458137 |
64 | P>R | No |
ClinGen ExAC gnomAD |
|
|
CA362163500 rs1202883517 |
66 | L>F | No |
ClinGen gnomAD |
|
|
CA362163481 rs1459934714 |
69 | L>V | No |
ClinGen gnomAD |
|
|
CA3563828 rs772542981 |
71 | A>P | No |
ClinGen ExAC gnomAD |
|
|
rs772542981 CA362163472 |
71 | A>T | Variant assessed as Somatic; 0.0 impact. [NCI-TCGA] | No |
ClinGen ExAC NCI-TCGA gnomAD |
|
VAR_069058 rs201362118 CA3563827 |
74 | G>D | No |
ClinGen UniProt 1000Genomes ExAC dbSNP gnomAD |
|
|
CA362163449 rs1216673146 |
75 | R>C | No |
ClinGen gnomAD |
|
|
CA362163446 COSM1436061 rs1363174603 |
75 | R>H | Variant assessed as Somatic; 0.0 impact. large_intestine [NCI-TCGA, Cosmic] | No |
ClinGen cosmic curated NCI-TCGA gnomAD |
|
CA3563825 rs771593440 |
77 | P>S | No |
ClinGen ExAC gnomAD |
|
|
rs749763672 CA3563824 |
78 | S>P | No |
ClinGen ExAC gnomAD |
|
|
rs777921797 CA3563821 |
79 | P>R | No |
ClinGen ExAC TOPMed gnomAD |
|
|
rs1337440208 CA362163425 |
79 | P>S | No |
ClinGen gnomAD |
|
|
rs1172891428 CA362163411 |
81 | K>N | No |
ClinGen gnomAD |
|
|
CA3563819 rs150813574 |
82 | C>S | No |
ClinGen 1000Genomes ExAC gnomAD |
|
|
CA362163407 rs1333780727 |
82 | C>Y | No |
ClinGen TOPMed |
|
|
CA3563818 RCV000520166 rs750249799 |
83 | A>T | No |
ClinGen ClinVar ExAC dbSNP gnomAD |
|
|
CA3563817 rs764967109 |
84 | S>C | No |
ClinGen ExAC gnomAD |
|
|
rs373807012 CA3563815 |
86 | F>S | No |
ClinGen ESP ExAC TOPMed gnomAD |
|
|
rs1352813413 CA362163377 |
87 | P>S | No |
ClinGen TOPMed |
|
|
CA362163373 rs1206097183 |
88 | A>T | No |
ClinGen gnomAD |
|
| TCGA novel | 89 | A>S | Variant assessed as Somatic; impact. [NCI-TCGA] | No | NCI-TCGA |
|
rs1169653363 CA362163356 |
90 | P>L | No |
ClinGen gnomAD |
|
|
CA362163361 rs1307727464 |
90 | P>T | No |
ClinGen gnomAD |
|
|
rs1314752131 CA362163354 |
91 | A>P | No |
ClinGen gnomAD |
|
|
rs1244289450 CA362163326 |
95 | R>C | Variant assessed as Somatic; 0.0 impact. [NCI-TCGA] | No |
ClinGen NCI-TCGA gnomAD |
|
CA362163324 rs763729448 |
95 | R>H | No |
ClinGen ExAC gnomAD |
|
|
CA362163304 rs1394343969 |
98 | S>R | No |
ClinGen gnomAD |
|
|
rs1365219269 CA362163290 |
101 | D>N | Variant assessed as Somatic; 0.0 impact. [NCI-TCGA] | No |
ClinGen NCI-TCGA gnomAD |
|
CA3563808 rs774878026 |
104 | K>N | No |
ClinGen ExAC TOPMed gnomAD |
|
|
rs1188239387 CA362163243 |
106 | P>L | No |
ClinGen gnomAD |
|
|
rs1188239387 CA362163241 |
106 | P>R | No |
ClinGen gnomAD |
|
|
CA3563807 rs771362938 |
107 | R>S | No |
ClinGen ExAC gnomAD |
|
|
COSM40378 rs749816778 CA3563806 |
108 | A>T | central_nervous_system [Cosmic] | No |
ClinGen cosmic curated ExAC gnomAD |
|
rs1290647391 CA362163206 |
109 | E>K | No |
ClinGen TOPMed gnomAD |
|
| TCGA novel | 114 | C>Y | Variant assessed as Somatic; impact. [NCI-TCGA] | No | NCI-TCGA |
|
CA362162049 rs1483052303 |
115 | A>P | No |
ClinGen TOPMed |
|
|
CA3563719 rs529610517 |
115 | A>V | No |
ClinGen 1000Genomes ExAC TOPMed gnomAD |
|
|
CA132259412 rs112167223 |
116 | L>R | No |
ClinGen Ensembl |
|
|
CA362162023 rs369025518 |
119 | A>G | No |
ClinGen ESP ExAC TOPMed gnomAD |
|
|
CA362162025 rs137852684 |
119 | A>T | Hypothyroidism, congenital, nongoitrous, 5 (chng5) [Ensembl] | No |
ClinGen 1000Genomes ESP ExAC TOPMed gnomAD |
|
CA3563718 rs369025518 |
119 | A>V | No |
ClinGen ESP ExAC TOPMed gnomAD |
|
|
rs913442600 CA132259393 |
120 | V>E | No |
ClinGen Ensembl |
|
|
CA362162021 rs1488724798 |
120 | V>L | No |
ClinGen TOPMed gnomAD |
|
|
CA362162020 rs1488724798 |
120 | V>M | No |
ClinGen TOPMed gnomAD |
|
|
CA362162005 rs1241311366 |
122 | L>P | No |
ClinGen TOPMed gnomAD |
|
|
CA132259390 rs990310551 |
123 | E>D | No |
ClinGen TOPMed gnomAD |
|
|
rs1466216124 CA362162003 |
123 | E>Q | No |
ClinGen gnomAD |
|
|
rs781260821 CA3563714 |
124 | K>E | No |
ClinGen ExAC TOPMed gnomAD |
|
|
CA362161973 rs387906774 |
127 | A>G | No |
ClinGen TOPMed gnomAD |
|
|
rs1220171192 CA362161976 |
127 | A>T | No |
ClinGen gnomAD |
|
|
rs779663474 CA3563711 |
128 | D>H | No |
ClinGen ExAC gnomAD |
|
|
rs779663474 CA3563712 |
128 | D>N | No |
ClinGen ExAC gnomAD |
|
|
CA3563710 rs758000160 |
129 | N>D | No |
ClinGen ExAC gnomAD |
|
|
CA3563708 rs765590955 |
131 | E>V | No |
ClinGen ExAC gnomAD |
|
|
rs1581108803 CA362161937 |
133 | P>H | No |
ClinGen Ensembl |
|
|
rs1182433334 CA362161933 |
134 | R>P | No |
ClinGen gnomAD |
|
|
rs1248623506 CA362161935 |
134 | R>W | No |
ClinGen gnomAD |
|
|
CA3563705 rs764551904 |
135 | A>E | No |
ClinGen ExAC gnomAD |
|
|
RCV000521992 rs1554093512 |
135 | A>missing | No |
ClinVar dbSNP |
|
|
CA362161925 rs1257246534 |
136 | R>* | No |
ClinGen gnomAD |
|
|
rs866619868 CA132259359 |
136 | R>L | No |
ClinGen TOPMed gnomAD |
|
|
rs866619868 CA362161924 |
136 | R>Q | Variant assessed as Somatic; impact. [NCI-TCGA] | No |
ClinGen NCI-TCGA TOPMed gnomAD |
|
CA362161920 rs761127819 |
137 | R>P | No |
ClinGen ExAC gnomAD |
|
|
rs761127819 CA3563704 |
137 | R>Q | No |
ClinGen ExAC gnomAD |
|
|
CA362161921 rs1260214548 |
137 | R>W | No |
ClinGen gnomAD |
|
|
CA362161915 rs1366528649 |
138 | R>Q | Variant assessed as Somatic; 0.0 impact. [NCI-TCGA] | No |
ClinGen NCI-TCGA gnomAD |
|
CA3563701 rs759682128 |
139 | R>K | No |
ClinGen ExAC |
|
|
rs1253114866 CA362161903 |
140 | K>R | No |
ClinGen TOPMed |
|
|
rs774627537 CA3563700 |
141 | P>A | No |
ClinGen ExAC gnomAD |
|
|
CA362161895 rs1303521925 |
141 | P>L | No |
ClinGen TOPMed gnomAD |
|
|
CA362161897 rs1303521925 |
141 | P>Q | No |
ClinGen TOPMed gnomAD |
|
|
rs1353021443 CA362161888 |
143 | V>M | No |
ClinGen gnomAD |
|
|
CA3563698 rs747932354 |
144 | L>R | No |
ClinGen ExAC gnomAD |
|
|
rs758277832 CA132259310 |
145 | F>L | No |
ClinGen Ensembl |
|
|
CA132259314 rs72554027 |
145 | F>S | No |
ClinGen TOPMed gnomAD |
|
|
CA362161871 rs1425850321 |
146 | S>T | No |
ClinGen TOPMed |
|
|
rs397516909 CA135152 RCV000037966 |
146 | S>W | No |
ClinGen ClinVar Ensembl dbSNP |
|
|
CA362161861 rs1442713675 |
147 | Q>L | No |
ClinGen gnomAD |
|
|
RCV000223727 rs876661381 |
148 | A>missing | No |
ClinVar dbSNP |
|
|
CA362161854 rs864321649 |
148 | A>V | Variant assessed as Somatic; 0.0 impact. [NCI-TCGA] | No |
ClinGen NCI-TCGA gnomAD |
|
CA3563695 rs746885826 |
151 | Y>C | No |
ClinGen ExAC gnomAD |
|
|
rs1244358587 CA362161826 |
152 | E>D | No |
ClinGen TOPMed gnomAD |
|
|
CA362161831 rs1291524589 |
152 | E>K | No |
ClinGen gnomAD |
|
|
rs1356254262 CA362161825 |
153 | L>M | No |
ClinGen TOPMed |
|
|
CA362161818 rs1324062610 |
154 | E>Q | Variant assessed as Somatic; 0.0 impact. [NCI-TCGA] | No |
ClinGen NCI-TCGA TOPMed gnomAD |
|
CA132259290 rs1018993104 |
155 | R>Q | No |
ClinGen gnomAD |
|
|
CA362161807 COSM1217397 rs1310163851 |
156 | R>C | Variant assessed as Somatic; 0.0 impact. large_intestine [NCI-TCGA, Cosmic] | No |
ClinGen cosmic curated NCI-TCGA gnomAD |
|
RCV000413858 CA16042518 rs1057518548 |
158 | K>N | No |
ClinGen ClinVar Ensembl dbSNP |
|
|
CA3563694 rs779716505 |
159 | Q>E | No |
ClinGen ExAC |
|
|
rs1299817619 CA362161783 |
159 | Q>H | No |
ClinGen gnomAD |
|
|
rs1410674749 CA362161785 |
159 | Q>R | No |
ClinGen gnomAD |
|
|
rs797045790 RCV000195107 RCV000484660 |
160 | Q>missing | No |
ClinVar dbSNP |
|
|
CA3563693 rs757925015 |
160 | Q>H | No |
ClinGen ExAC gnomAD |
|
|
CA3563691 rs757605578 |
163 | L>P | No |
ClinGen ExAC gnomAD |
|
|
CA362161755 rs1554093487 RCV000621937 |
164 | S>* | No |
ClinGen ClinVar Ensembl dbSNP |
|
|
rs984722259 CA132259258 |
165 | A>V | No |
ClinGen TOPMed gnomAD |
|
|
rs1183825086 CA362161742 |
166 | P>L | No |
ClinGen gnomAD |
|
|
CA3563688 rs756623702 |
169 | D>G | No |
ClinGen ExAC TOPMed gnomAD |
|
|
CA3563689 rs764390691 |
169 | D>Y | No |
ClinGen ExAC gnomAD |
|
|
rs1489743522 CA362161716 |
170 | Q>H | No |
ClinGen TOPMed gnomAD |
|
|
rs786205825 RCV000171015 |
170 | Q>missing | No |
ClinVar dbSNP |
|
|
rs797045791 CA206131 RCV000192960 |
171 | L>R | No |
ClinGen ClinVar Ensembl dbSNP |
|
|
CA362161704 rs1298345224 |
173 | S>G | No |
ClinGen TOPMed |
|
|
CA362161697 rs1238421153 |
174 | V>L | No |
ClinGen TOPMed gnomAD |
|
|
rs1238421153 CA362161696 |
174 | V>M | No |
ClinGen TOPMed gnomAD |
|
|
rs3729938 CA132259223 |
179 | S>C | No |
ClinGen Ensembl |
|
|
CA362161657 rs1256674245 |
180 | T>M | No |
ClinGen TOPMed |
|
|
rs1345146178 CA362161654 |
181 | Q>* | No |
ClinGen gnomAD |
|
|
rs1384912539 CA362161649 |
182 | V>I | No |
ClinGen gnomAD |
|
|
rs786205826 RCV000171016 |
185 | W>missing | No |
ClinVar dbSNP |
|
|
RCV000193839 rs797045792 CA207589 |
185 | W>L | No |
ClinGen ClinVar Ensembl dbSNP |
|
|
rs1581108437 RCV001008425 |
186 | F>missing | No |
ClinVar dbSNP |
|
|
CA362161571 rs1428226457 |
193 | C>R | No |
ClinGen gnomAD |
|
|
rs1197189103 CA362161569 |
193 | C>Y | No |
ClinGen gnomAD |
|
|
CA132259180 rs371694530 |
196 | Q>* | No |
ClinGen ESP |
|
|
rs774482632 RCV000519325 CA362161542 |
197 | R>Q | No |
ClinGen ClinVar ExAC TOPMed dbSNP |
|
|
rs763219076 CA3563684 |
200 | Q>K | No |
ClinGen ExAC gnomAD |
|
|
rs776582660 CA3563683 |
202 | L>M | No |
ClinGen ExAC TOPMed gnomAD |
|
|
rs776582660 CA362161512 |
202 | L>V | No |
ClinGen ExAC TOPMed gnomAD |
|
|
CA3563681 rs771533553 |
203 | E>A | No |
ClinGen ExAC TOPMed gnomAD |
|
|
CA362161501 rs1386410484 |
204 | L>P | No |
ClinGen gnomAD |
|
|
CA362161498 rs1366026905 |
205 | V>M | No |
ClinGen TOPMed |
|
|
rs1457368487 CA362161489 |
206 | G>E | No |
ClinGen gnomAD |
|
|
CA362161479 rs1226704953 |
208 | P>S | Variant assessed as Somatic; impact. [NCI-TCGA] | No |
ClinGen NCI-TCGA TOPMed |
|
rs1267723705 CA362161470 |
209 | P>R | No |
ClinGen TOPMed |
|
| TCGA novel | 210 | P>L | Variant assessed as Somatic; impact. [NCI-TCGA] | No | NCI-TCGA |
|
rs372282873 CA3563676 |
212 | P>L | No |
ClinGen ESP ExAC TOPMed gnomAD |
|
|
CA209605 rs372282873 RCV000195044 |
212 | P>Q | No |
ClinGen ClinVar ESP ExAC TOPMed dbSNP gnomAD |
|
|
rs753261087 CA3563675 |
213 | P>L | No |
ClinGen ExAC gnomAD |
|
|
rs781707428 CA3563674 |
214 | P>L | No |
ClinGen ExAC TOPMed gnomAD |
|
| TCGA novel | 215 | A>P | Variant assessed as Somatic; impact. [NCI-TCGA] | No | NCI-TCGA |
|
CA362161440 rs1161668727 |
215 | A>V | No |
ClinGen TOPMed |
|
|
rs1201267220 CA362161436 |
216 | R>H | No |
ClinGen gnomAD |
|
|
CA132259068 rs888454270 |
218 | I>T | No |
ClinGen TOPMed gnomAD |
|
|
CA362161399 rs1435738199 |
223 | L>V | No |
ClinGen gnomAD |
|
| TCGA novel | 225 | R>H | Variant assessed as Somatic; impact. [NCI-TCGA] | No | NCI-TCGA |
|
rs929243588 CA132259055 |
226 | D>G | No |
ClinGen TOPMed |
|
|
rs775331755 CA3563663 |
227 | G>D | No |
ClinGen ExAC TOPMed gnomAD |
|
| TCGA novel | 227 | G>V | Variant assessed as Somatic; impact. [NCI-TCGA] | No | NCI-TCGA |
|
CA3563662 rs772081524 |
228 | K>Q | No |
ClinGen ExAC gnomAD |
|
|
rs922066386 CA132259045 |
228 | K>T | No |
ClinGen Ensembl |
|
|
RCV000175778 rs797044675 |
230 | C>missing | No |
ClinVar dbSNP |
|
|
CA362161352 rs1428281500 |
231 | L>V | No |
ClinGen gnomAD |
|
|
rs759339072 CA3563661 |
232 | G>A | No |
ClinGen ExAC gnomAD |
|
|
CA362161348 rs1225707941 |
232 | G>R | No |
ClinGen TOPMed |
|
|
CA362161343 rs1222755872 |
233 | D>N | Variant assessed as Somatic; impact. [NCI-TCGA] | No |
ClinGen NCI-TCGA TOPMed gnomAD |
|
RCV000428074 rs773922431 CA16605325 |
234 | S>* | Variant assessed as Somatic; impact. [NCI-TCGA] | No |
ClinGen ClinVar ExAC NCI-TCGA TOPMed dbSNP gnomAD |
|
rs773922431 CA3563660 |
234 | S>L | No |
ClinGen ExAC TOPMed gnomAD |
|
|
rs773922431 CA362161332 |
234 | S>W | No |
ClinGen ExAC TOPMed gnomAD |
|
|
rs749004208 CA3563658 |
235 | A>V | No |
ClinGen ExAC TOPMed gnomAD |
|
| TCGA novel | 238 | A>T | Variant assessed as Somatic; impact. [NCI-TCGA] | No | NCI-TCGA |
|
CA362161309 rs1470284025 |
238 | A>V | No |
ClinGen TOPMed |
|
|
rs866024579 CA132259020 |
242 | G>D | No |
ClinGen Ensembl |
|
|
rs781370140 CA3563655 |
243 | V>M | No |
ClinGen ExAC gnomAD |
|
|
CA362161274 rs1581108127 |
244 | G>V | No |
ClinGen Ensembl |
|
|
rs966145309 CA132258999 |
246 | N>S | No |
ClinGen gnomAD |
|
|
rs780348618 CA3563652 |
247 | P>A | No |
ClinGen ExAC gnomAD |
|
|
rs780348618 CA3563653 |
247 | P>T | No |
ClinGen ExAC gnomAD |
|
|
rs750726737 CA3563650 |
249 | G>D | No |
ClinGen ExAC TOPMed gnomAD |
|
|
rs919084655 CA132258989 |
249 | G>S | No |
ClinGen TOPMed |
|
|
CA362161240 rs1289763464 |
250 | Y>C | No |
ClinGen Ensembl |
|
|
CA3563647 rs752557173 |
253 | Y>S | No |
ClinGen ExAC gnomAD |
|
| TCGA novel | 255 | A>S | Variant assessed as Somatic; impact. [NCI-TCGA] | No | NCI-TCGA |
|
rs759518211 CA3563645 |
255 | A>T | No |
ClinGen ExAC gnomAD |
|
|
rs1165063212 CA362161206 |
256 | Y>N | No |
ClinGen gnomAD |
|
|
rs770484529 CA3563643 |
257 | P>L | No |
ClinGen ExAC gnomAD |
|
|
rs387906776 CA3563644 |
257 | P>S | Ventricular septal defect 3 (vsd3) [Ensembl] | No |
ClinGen ExAC TOPMed gnomAD |
|
rs772889193 CA3563641 |
258 | G>C | No |
ClinGen ExAC TOPMed gnomAD |
|
|
rs772889193 CA362161197 |
258 | G>S | No |
ClinGen ExAC TOPMed gnomAD |
|
|
rs1057520670 RCV000432384 CA16605324 |
259 | Y>* | No |
ClinGen ClinVar Ensembl dbSNP |
|
|
CA3563640 rs553883993 |
259 | Y>F | No |
ClinGen 1000Genomes ExAC TOPMed gnomAD |
|
|
CA3563639 rs747698510 |
262 | A>S | No |
ClinGen ExAC TOPMed gnomAD |
|
|
rs1450940026 CA362161171 |
262 | A>V | No |
ClinGen gnomAD |
|
|
CA3563638 rs777059602 |
264 | C>R | No |
ClinGen ExAC gnomAD |
|
|
rs1229530745 CA362161162 |
264 | C>Y | No |
ClinGen gnomAD |
|
|
CA3563635 rs780688010 |
267 | G>R | No |
ClinGen ExAC TOPMed gnomAD |
|
|
CA3563634 rs758595778 |
269 | S>G | No |
ClinGen ExAC gnomAD |
|
| TCGA novel | 269 | S>I | Variant assessed as Somatic; impact. [NCI-TCGA] | No | NCI-TCGA |
|
CA362161128 rs1289353345 |
269 | S>T | No |
ClinGen gnomAD |
|
|
rs994687523 CA132258862 |
271 | T>A | Variant assessed as Somatic; impact. [NCI-TCGA] | No |
ClinGen Ensembl NCI-TCGA |
|
rs779089768 CA3563633 |
273 | A>P | No |
ClinGen ExAC gnomAD |
|
|
CA3563632 rs757511605 |
273 | A>V | No |
ClinGen ExAC gnomAD |
|
|
CA3563631 rs753938777 |
274 | Y>S | No |
ClinGen ExAC |
|
|
CA362161095 rs368366482 |
275 | P>A | No |
ClinGen 1000Genomes ESP ExAC TOPMed gnomAD |
|
|
CA362161094 rs368366482 |
275 | P>S | Variant assessed as Somatic; 0.0 impact. [NCI-TCGA] | No |
ClinGen 1000Genomes ESP ExAC NCI-TCGA TOPMed gnomAD |
|
CA362161091 rs1425022333 |
276 | A>T | No |
ClinGen gnomAD |
|
|
rs772729751 CA362161085 |
277 | G>R | No |
ClinGen ExAC gnomAD |
|
|
rs772729751 CA3563625 |
277 | G>W | No |
ClinGen ExAC gnomAD |
|
|
CA3563624 rs571382279 |
279 | S>A | No |
ClinGen 1000Genomes ExAC gnomAD |
|
|
CA362161063 rs1266892994 |
281 | A>S | No |
ClinGen gnomAD |
|
|
rs1266892994 CA362161065 |
281 | A>T | No |
ClinGen gnomAD |
|
|
rs375086983 CA362161047 |
283 | P>L | Ventricular septal defect 3 (vsd3) [Ensembl] | No |
ClinGen ExAC TOPMed gnomAD |
|
CA132258815 rs988348581 |
284 | A>T | No |
ClinGen TOPMed |
|
|
CA362161040 rs1188424210 |
285 | T>A | No |
ClinGen gnomAD |
|
|
rs746315645 CA3563618 |
288 | A>T | Variant assessed as Somatic; 0.0 impact. [NCI-TCGA] | No |
ClinGen ExAC NCI-TCGA gnomAD |
| TCGA novel | 289 | N>K | Variant assessed as Somatic; impact. [NCI-TCGA] | No | NCI-TCGA |
|
CA3563617 rs779136385 |
289 | N>K | No |
ClinGen ExAC gnomAD |
|
|
rs1318592912 CA362161001 |
291 | N>T | No |
ClinGen gnomAD |
|
|
VAR_067588 rs756974215 |
291 | N>del | CTMH [UniProt] | No |
UniProt dbSNP |
|
CA132258782 rs538010963 |
292 | F>L | No |
ClinGen 1000Genomes TOPMed |
|
|
rs757275107 CA3563616 |
292 | F>V | No |
ClinGen ExAC |
|
|
rs749577978 CA3563615 |
293 | V>L | No |
ClinGen ExAC gnomAD |
|
|
rs373421818 CA3563611 RCV000617167 |
296 | G>D | No |
ClinGen ClinVar ESP ExAC TOPMed dbSNP gnomAD |
|
| TCGA novel | 297 | V>D | Variant assessed as Somatic; impact. [NCI-TCGA] | No | NCI-TCGA |
|
rs549406766 CA3563607 |
298 | G>E | No |
ClinGen 1000Genomes ExAC gnomAD |
|
|
rs763688050 CA3563604 CA3563605 |
301 | N>K | No |
ClinGen ExAC TOPMed gnomAD |
|
|
rs371380388 CA3563603 |
302 | A>E | No |
ClinGen ExAC gnomAD |
|
|
CA362160894 COSM1217396 rs371380388 |
302 | A>V | large_intestine [Cosmic] | No |
ClinGen cosmic curated ExAC gnomAD |
|
CA362160878 rs1310111410 |
304 | Q>E | No |
ClinGen gnomAD |
|
|
rs774983960 CA3563599 |
306 | P>H | No |
ClinGen ExAC gnomAD |
|
|
CA3563598 rs771088497 |
308 | I>T | No |
ClinGen ExAC TOPMed gnomAD |
|
|
rs142368156 CA3563597 |
311 | S>N | No |
ClinGen ESP ExAC gnomAD |
|
|
CA3563595 rs769930017 RCV000213088 |
313 | S>L | No |
ClinGen ClinVar ExAC dbSNP gnomAD |
|
|
rs777867699 CA3563596 |
313 | S>P | No |
ClinGen ExAC gnomAD |
|
|
rs200152391 CA3563592 |
314 | G>A | No |
ClinGen 1000Genomes ESP ExAC TOPMed gnomAD |
|
|
CA3563593 rs200152391 |
314 | G>E | No |
ClinGen 1000Genomes ESP ExAC TOPMed gnomAD |
|
|
CA3563591 rs201249977 |
315 | V>M | No |
ClinGen 1000Genomes ESP ExAC TOPMed gnomAD |
|
|
RCV000620703 rs1196710127 CA362160716 RCV001756005 |
319 | H>R | No |
ClinGen ClinVar dbSNP gnomAD |
|
|
CA132258701 rs944504713 |
321 | I>V | No |
ClinGen TOPMed gnomAD |
|
|
rs376426882 CA3563588 |
322 | R>P | No |
ClinGen ESP ExAC gnomAD |
|
|
rs1188394387 CA362160674 |
323 | A>D | No |
ClinGen TOPMed |
6 associated diseases with P52952
[MIM: 108900]: Atrial septal defect 7, with or without atrioventricular conduction defects (ASD7)
A congenital heart malformation characterized by incomplete closure of the wall between the atria resulting in blood flow from the left to the right atria, and atrioventricular conduction defects in some cases. {ECO:0000269|PubMed:10587520, ECO:0000269|PubMed:14607454, ECO:0000269|PubMed:15342699, ECO:0000269|PubMed:15810002, ECO:0000269|PubMed:9651244}. Note=The disease is caused by variants affecting the gene represented in this entry.
[MIM: 187500]: Tetralogy of Fallot (TOF)
A congenital heart anomaly which consists of pulmonary stenosis, ventricular septal defect, dextroposition of the aorta (aorta is on the right side instead of the left) and hypertrophy of the right ventricle. In this condition, blood from both ventricles (oxygen-rich and oxygen-poor) is pumped into the body often causing cyanosis. {ECO:0000269|PubMed:10587520, ECO:0000269|PubMed:11714651, ECO:0000269|PubMed:14607454}. Note=The disease is caused by variants affecting the gene represented in this entry.
[MIM: 217095]: Conotruncal heart malformations (CTHM)
A group of congenital heart defects involving the outflow tracts. Examples include truncus arteriosus communis, double-outlet right ventricle and transposition of great arteries. Truncus arteriosus communis is characterized by a single outflow tract instead of a separate aorta and pulmonary artery. In transposition of the great arteries, the aorta arises from the right ventricle and the pulmonary artery from the left ventricle. In double outlet of the right ventricle, both the pulmonary artery and aorta arise from the right ventricle. {ECO:0000269|PubMed:14607454, ECO:0000269|PubMed:17891434}. Note=The disease is caused by variants affecting the gene represented in this entry.
[MIM: 225250]: Hypothyroidism, congenital, non-goitrous, 5 (CHNG5)
A non-autoimmune condition characterized by resistance to thyroid-stimulating hormone (TSH) leading to increased levels of plasma TSH and low levels of thyroid hormone. CHNG5 presents variable severity depending on the completeness of the defect. Most patients are euthyroid and asymptomatic, with a normal sized thyroid gland. Only a subset of patients develop hypothyroidism and present a hypoplastic thyroid gland. {ECO:0000269|PubMed:16418214}. Note=The disease is caused by variants affecting the gene represented in this entry.
[MIM: 614432]: Ventricular septal defect 3 (VSD3)
A common form of congenital cardiovascular anomaly that may occur alone or in combination with other cardiac malformations. It can affect any portion of the ventricular septum, resulting in abnormal communications between the two lower chambers of the heart. Classification is based on location of the communication, such as perimembranous, inlet, outlet (infundibular), central muscular, marginal muscular, or apical muscular defect. Large defects that go unrepaired may give rise to cardiac enlargement, congestive heart failure, pulmonary hypertension, Eisenmenger's syndrome, delayed fetal brain development, arrhythmias, and even sudden cardiac death. {ECO:0000269|PubMed:21110066, ECO:0000269|PubMed:21165553}. Note=The disease is caused by variants affecting the gene represented in this entry.
[MIM: 614435]: Hypoplastic left heart syndrome 2 (HLHS2)
A syndrome due to defective development of the aorta proximal to the entrance of the ductus arteriosus, and hypoplasia of the left ventricle and mitral valve. As a result of the abnormal circulation, the ductus arteriosus and foramen ovale are patent and the right atrium, right ventricle, and pulmonary artery are enlarged. {ECO:0000269|PubMed:14607454, ECO:0000269|PubMed:15810002}. Note=The disease is caused by variants affecting the gene represented in this entry.
Without disease ID
- A congenital heart malformation characterized by incomplete closure of the wall between the atria resulting in blood flow from the left to the right atria, and atrioventricular conduction defects in some cases. {ECO:0000269|PubMed:10587520, ECO:0000269|PubMed:14607454, ECO:0000269|PubMed:15342699, ECO:0000269|PubMed:15810002, ECO:0000269|PubMed:9651244}. Note=The disease is caused by variants affecting the gene represented in this entry.
- A congenital heart anomaly which consists of pulmonary stenosis, ventricular septal defect, dextroposition of the aorta (aorta is on the right side instead of the left) and hypertrophy of the right ventricle. In this condition, blood from both ventricles (oxygen-rich and oxygen-poor) is pumped into the body often causing cyanosis. {ECO:0000269|PubMed:10587520, ECO:0000269|PubMed:11714651, ECO:0000269|PubMed:14607454}. Note=The disease is caused by variants affecting the gene represented in this entry.
- A group of congenital heart defects involving the outflow tracts. Examples include truncus arteriosus communis, double-outlet right ventricle and transposition of great arteries. Truncus arteriosus communis is characterized by a single outflow tract instead of a separate aorta and pulmonary artery. In transposition of the great arteries, the aorta arises from the right ventricle and the pulmonary artery from the left ventricle. In double outlet of the right ventricle, both the pulmonary artery and aorta arise from the right ventricle. {ECO:0000269|PubMed:14607454, ECO:0000269|PubMed:17891434}. Note=The disease is caused by variants affecting the gene represented in this entry.
- A non-autoimmune condition characterized by resistance to thyroid-stimulating hormone (TSH) leading to increased levels of plasma TSH and low levels of thyroid hormone. CHNG5 presents variable severity depending on the completeness of the defect. Most patients are euthyroid and asymptomatic, with a normal sized thyroid gland. Only a subset of patients develop hypothyroidism and present a hypoplastic thyroid gland. {ECO:0000269|PubMed:16418214}. Note=The disease is caused by variants affecting the gene represented in this entry.
- A common form of congenital cardiovascular anomaly that may occur alone or in combination with other cardiac malformations. It can affect any portion of the ventricular septum, resulting in abnormal communications between the two lower chambers of the heart. Classification is based on location of the communication, such as perimembranous, inlet, outlet (infundibular), central muscular, marginal muscular, or apical muscular defect. Large defects that go unrepaired may give rise to cardiac enlargement, congestive heart failure, pulmonary hypertension, Eisenmenger's syndrome, delayed fetal brain development, arrhythmias, and even sudden cardiac death. {ECO:0000269|PubMed:21110066, ECO:0000269|PubMed:21165553}. Note=The disease is caused by variants affecting the gene represented in this entry.
- A syndrome due to defective development of the aorta proximal to the entrance of the ductus arteriosus, and hypoplasia of the left ventricle and mitral valve. As a result of the abnormal circulation, the ductus arteriosus and foramen ovale are patent and the right atrium, right ventricle, and pulmonary artery are enlarged. {ECO:0000269|PubMed:14607454, ECO:0000269|PubMed:15810002}. Note=The disease is caused by variants affecting the gene represented in this entry.
4 regional properties for P52952
| Type | Name | Position | InterPro Accession |
|---|---|---|---|
| domain | Homeobox domain | 136 - 200 | IPR001356 |
| conserved_site | Homeobox, conserved site | 171 - 194 | IPR017970 |
| domain | Homeobox domain, metazoa | 160 - 171 | IPR020479-1 |
| domain | Homeobox domain, metazoa | 175 - 194 | IPR020479-2 |
7 GO annotations of cellular component
| Name | Definition |
|---|---|
| chromatin | The ordered and organized complex of DNA, protein, and sometimes RNA, that forms the chromosome. |
| cytoplasm | The contents of a cell excluding the plasma membrane and nucleus, but including other subcellular structures. |
| nucleus | A membrane-bounded organelle of eukaryotic cells in which chromosomes are housed and replicated. In most cells, the nucleus contains all of the cell's chromosomes except the organellar chromosomes, and is the site of RNA synthesis and processing. In some species, or in specialized cell types, RNA metabolism or DNA replication may be absent. |
| protein-containing complex | A stable assembly of two or more macromolecules, i.e. proteins, nucleic acids, carbohydrates or lipids, in which at least one component is a protein and the constituent parts function together. |
| protein-DNA complex | A macromolecular complex containing both protein and DNA molecules. |
| RNA polymerase II transcription regulator complex | A transcription factor complex that acts at a regulatory region of a gene transcribed by RNA polymerase II. |
| transcription regulator complex | A protein complex that is capable of associating with DNA by direct binding, or via other DNA-binding proteins or complexes, and regulating transcription. |
12 GO annotations of molecular function
| Name | Definition |
|---|---|
| chromatin binding | Binding to chromatin, the network of fibers of DNA, protein, and sometimes RNA, that make up the chromosomes of the eukaryotic nucleus during interphase. |
| DNA binding | Any molecular function by which a gene product interacts selectively and non-covalently with DNA (deoxyribonucleic acid). |
| DNA-binding transcription activator activity | A DNA-binding transcription factor activity that activates or increases transcription of specific gene sets. |
| DNA-binding transcription activator activity, RNA polymerase II-specific | A DNA-binding transcription factor activity that activates or increases transcription of specific gene sets transcribed by RNA polymerase II. |
| DNA-binding transcription factor activity | A transcription regulator activity that modulates transcription of gene sets via selective and non-covalent binding to a specific double-stranded genomic DNA sequence (sometimes referred to as a motif) within a cis-regulatory region. Regulatory regions include promoters (proximal and distal) and enhancers. Genes are transcriptional units, and include bacterial operons. |
| DNA-binding transcription factor activity, RNA polymerase II-specific | A DNA-binding transcription factor activity that modulates the transcription of specific gene sets transcribed by RNA polymerase II. |
| protein homodimerization activity | Binding to an identical protein to form a homodimer. |
| RNA polymerase II cis-regulatory region sequence-specific DNA binding | Binding to a specific upstream regulatory DNA sequence (transcription factor recognition sequence or binding site) located in cis relative to the transcription start site (i.e., on the same strand of DNA) of a gene transcribed by RNA polymerase II. |
| RNA polymerase II-specific DNA-binding transcription factor binding | Binding to a sequence-specific DNA binding RNA polymerase II transcription factor, any of the factors that interact selectively and non-covalently with a specific DNA sequence in order to modulate transcription. |
| sequence-specific DNA binding | Binding to DNA of a specific nucleotide composition, e.g. GC-rich DNA binding, or with a specific sequence motif or type of DNA e.g. promotor binding or rDNA binding. |
| sequence-specific double-stranded DNA binding | Binding to double-stranded DNA of a specific nucleotide composition, e.g. GC-rich DNA binding, or with a specific sequence motif or type of DNA, e.g. promotor binding or rDNA binding. |
| transcription cis-regulatory region binding | Binding to a specific sequence of DNA that is part of a regulatory region that controls transcription of that section of the DNA. The transcribed region might be described as a gene, cistron, or operon. |
63 GO annotations of biological process
| Name | Definition |
|---|---|
| adult heart development | The process whose specific outcome is the progression of the adult heart over time, from its formation to the mature structure. |
| aortic valve morphogenesis | The process in which the structure of the aortic valve is generated and organized. |
| apoptotic process involved in heart morphogenesis | Any apoptotic process that contributes to the shaping of the heart. |
| atrial cardiac muscle cell development | The process whose specific outcome is the progression of an atrial cardiac muscle cell over time, from its formation to the mature state. Cardiac muscle cells are striated muscle cells that are responsible for heart contraction. The atrium is the part of the heart that receives blood into the organ. |
| atrial cardiac muscle tissue development | The process whose specific outcome is the progression of cardiac muscle of the atrium over time, from its formation to the mature structure. |
| atrial septum morphogenesis | The developmental process in which atrial septum is generated and organized. The atrial septum separates the upper chambers (the atria) of the heart from one another. |
| atrioventricular node cell development | The process whose specific outcome is the progression of an atrioventricular (AV) node cell over time, from its formation to the mature state. |
| atrioventricular node cell fate commitment | The commitment of cells to atrioventricular (AV) node cell fates and their capacity to differentiate into AV node cells. |
| atrioventricular node development | The process whose specific outcome is the progression of the atrioventricular (AV) node over time, from its formation to the mature structure. The AV node is part of the cardiac conduction system that controls the timing of ventricle contraction by receiving electrical signals from the sinoatrial (SA) node and relaying them to the His-Purkinje system. |
| bundle of His development | The process whose specific outcome is the progression of the bundle of His over time, from its formation to the mature structure. The bundle of His is part of the His-Purkinje system that transmits signals from the AV node to the cardiac Purkinje fibers. |
| cardiac conduction system development | The process whose specific outcome is the progression of the cardiac conduction system over time, from its formation to the mature structure. The cardiac conduction system consists of specialized cardiomyocytes that regulate the frequency of heart beat. |
| cardiac muscle cell development | The process whose specific outcome is the progression of a cardiac muscle cell over time, from its formation to the mature state. |
| cardiac muscle cell differentiation | The process in which a cardiac muscle precursor cell acquires specialized features of a cardiac muscle cell. Cardiac muscle cells are striated muscle cells that are responsible for heart contraction. |
| cardiac muscle cell proliferation | The expansion of a cardiac muscle cell population by cell division. |
| cardiac muscle contraction | Muscle contraction of cardiac muscle tissue. |
| cardiac muscle tissue morphogenesis | The process in which the anatomical structures of cardiac muscle tissue are generated and organized. |
| cardiac ventricle formation | The developmental process pertaining to the initial formation of a cardiac ventricle from unspecified parts. A cardiac ventricle receives blood from a cardiac atrium and pumps it out of the heart. |
| cell differentiation | The process in which relatively unspecialized cells, e.g. embryonic or regenerative cells, acquire specialized structural and/or functional features that characterize the cells, tissues, or organs of the mature organism or some other relatively stable phase of the organism's life history. Differentiation includes the processes involved in commitment of a cell to a specific fate and its subsequent development to the mature state. |
| embryonic heart tube development | The process whose specific outcome is the progression of the embryonic heart tube over time, from its formation to the mature structure. The heart tube forms as the heart rudiment from the heart field. |
| embryonic heart tube left/right pattern formation | The pattern specification process that results in the subdivision of the left/right axis of the embryonic heart tube in space to define an area or volume in which specific patterns of cell differentiation will take place. |
| epithelial cell apoptotic process | Any apoptotic process in an epithelial cell. |
| epithelial cell differentiation | The process in which a relatively unspecialized cell acquires specialized features of an epithelial cell, any of the cells making up an epithelium. |
| epithelial cell proliferation | The multiplication or reproduction of epithelial cells, resulting in the expansion of a cell population. Epithelial cells make up the epithelium, the covering of internal and external surfaces of the body, including the lining of vessels and other small cavities. It consists of cells joined by small amounts of cementing substances. |
| heart looping | The tube morphogenesis process in which the primitive heart tube loops asymmetrically. This looping brings the primitive heart chambers into alignment preceding their future integration. Heart looping begins with dextral-looping and ends when the main regional divisions of the mature heart and primordium of the great arterial trunks become established preceeding septation. |
| heart morphogenesis | The developmental process in which the heart is generated and organized. The heart is a hollow, muscular organ, which, by contracting rhythmically, keeps up the circulation of the blood. |
| heart trabecula formation | The process of creating a trabecula in the heart. A trabecula is a tissue element in the form of a small beam, strut or rod. |
| hemopoiesis | The process whose specific outcome is the progression of the myeloid and lymphoid derived organ/tissue systems of the blood and other parts of the body over time, from formation to the mature structure. The site of hemopoiesis is variable during development, but occurs primarily in bone marrow or kidney in many adult vertebrates. |
| negative regulation of apoptotic process | Any process that stops, prevents, or reduces the frequency, rate or extent of cell death by apoptotic process. |
| negative regulation of canonical Wnt signaling pathway | Any process that decreases the rate, frequency, or extent of the Wnt signaling pathway through beta-catenin, the series of molecular signals initiated by binding of a Wnt protein to a frizzled family receptor on the surface of the target cell, followed by propagation of the signal via beta-catenin, and ending with a change in transcription of target genes. |
| negative regulation of cardiac muscle cell apoptotic process | Any process that decreases the rate or extent of cardiac cell apoptotic process, a form of programmed cell death induced by external or internal signals that trigger the activity of proteolytic caspases whose actions dismantle a cardiac muscle cell and result in its death. |
| negative regulation of DNA-templated transcription | Any process that stops, prevents, or reduces the frequency, rate or extent of cellular DNA-templated transcription. |
| negative regulation of epithelial cell apoptotic process | Any process that stops, prevents or reduces the frequency, rate or extent of epithelial cell apoptotic process. |
| negative regulation of myotube differentiation | Any process that decreases the frequency, rate or extent of myotube differentiation. Myotube differentiation is the process in which a relatively unspecialized cell acquires specialized features of a myotube cell. Myotubes are multinucleated cells that are formed when proliferating myoblasts exit the cell cycle, differentiate and fuse. |
| negative regulation of transcription by RNA polymerase II | Any process that stops, prevents, or reduces the frequency, rate or extent of transcription mediated by RNA polymerase II. |
| outflow tract septum morphogenesis | The process in which the anatomical structures of the outflow tract septum are generated and organized. The outflow tract septum is a partition in the outflow tract. |
| pharyngeal system development | The process whose specific outcome is the progression of the pharyngeal system over time, from its formation to the mature structure. The pharyngeal system is a transient embryonic complex that is specific to vertebrates. It comprises the pharyngeal arches, bulges of tissues of mesoderm and neural crest derivation through which pass nerves and pharyngeal arch arteries. The arches are separated internally by pharyngeal pouches, evaginations of foregut endoderm, and externally by pharyngeal clefts, invaginations of surface ectoderm. The development of the system ends when the stucture it contributes to are forming: the thymus, thyroid, parathyroids, maxilla, mandible, aortic arch, cardiac outflow tract, external and middle ear. |
| positive regulation of cardioblast differentiation | Any process that activates or increases the frequency, rate or extent of cardioblast differentiation, the process in which a relatively unspecialized mesodermal cell acquires the specialized structural and/or functional features of a cardioblast. A cardioblast is a cardiac precursor cell. It is a cell that has been committed to a cardiac fate, but will undergo more cell division rather than terminally differentiating. |
| positive regulation of cell population proliferation | Any process that activates or increases the rate or extent of cell proliferation. |
| positive regulation of DNA-templated transcription | Any process that activates or increases the frequency, rate or extent of cellular DNA-templated transcription. |
| positive regulation of epithelial cell proliferation | Any process that activates or increases the rate or extent of epithelial cell proliferation. |
| positive regulation of gene expression | Any process that increases the frequency, rate or extent of gene expression. Gene expression is the process in which a gene's coding sequence is converted into a mature gene product (protein or RNA). |
| positive regulation of heart contraction | Any process that activates or increases the frequency, rate or extent of heart contraction. |
| positive regulation of neuron differentiation | Any process that activates or increases the frequency, rate or extent of neuron differentiation. |
| positive regulation of sodium ion transport | Any process that increases the frequency, rate or extent of the directed movement of sodium ions (Na+) into, out of or within a cell, or between cells, by means of some agent such as a transporter or pore. |
| positive regulation of transcription by RNA polymerase II | Any process that activates or increases the frequency, rate or extent of transcription from an RNA polymerase II promoter. |
| positive regulation of transcription initiation by RNA polymerase II | Any process that increases the rate, frequency or extent of a process involved in starting transcription from an RNA polymerase II promoter. |
| proepicardium development | The progression of the proepicardium from its formation to the mature structure. The proepicardium is an outpouching of the septum transversum. |
| pulmonary myocardium development | The progression of the pulmonary myocardium over time, from its initial formation to the mature structure. The pulmonary myocardium is the myocardial tissue present in the pulmonary vein. |
| Purkinje myocyte differentiation | The process in which a relatively unspecialized cell acquires the specialized structural and/or functional features of a Purkinje myocyte (also known as cardiac Purkinje fiber cell). These cells are specialized cardiomyocytes that receive signals from the bundle of His and innervate the ventricular cardiac muscle. |
| regulation of cardiac conduction | Any process that modulates the frequency, rate or extent of cardiac conduction. |
| regulation of cardiac muscle cell proliferation | Any process that modulates the frequency, rate or extent of cardiac muscle cell proliferation. |
| regulation of cardiac muscle contraction | Any process that modulates the frequency, rate or extent of cardiac muscle contraction. |
| regulation of transcription by RNA polymerase II | Any process that modulates the frequency, rate or extent of transcription mediated by RNA polymerase II. |
| right ventricular cardiac muscle tissue morphogenesis | The process in which the anatomical structures of the right cardiac ventricle muscle are generated and organized. |
| septum secundum development | The progression of the septum secundum over time, from its initial formation to the mature structure. |
| spleen development | The process whose specific outcome is the progression of the spleen over time, from its formation to the mature structure. The spleen is a large vascular lymphatic organ composed of white and red pulp, involved both in hemopoietic and immune system functions. |
| thyroid gland development | The process whose specific outcome is the progression of the thyroid gland over time, from its formation to the mature structure. The thyroid gland is an endoderm-derived gland that produces thyroid hormone. |
| transcription by RNA polymerase II | The synthesis of RNA from a DNA template by RNA polymerase II (RNAP II), originating at an RNA polymerase II promoter. Includes transcription of messenger RNA (mRNA) and certain small nuclear RNAs (snRNAs). |
| vasculogenesis | The differentiation of endothelial cells from progenitor cells during blood vessel development, and the de novo formation of blood vessels and tubes. |
| ventricular cardiac muscle cell development | The process whose specific outcome is the progression of a ventricular cardiac muscle cell over time, from its formation to the mature state. Cardiac muscle cells are striated muscle cells that are responsible for heart contraction. The ventricle is the part of the heart that pumps blood out of the organ. |
| ventricular cardiac myofibril assembly | The process whose specific outcome is the progression of the ventricular cardiac myofibril over time, from its formation to the mature structure. A cardiac myofibril is a myofibril specific to cardiac muscle cells. |
| ventricular septum morphogenesis | The developmental process in which a ventricular septum is generated and organized. A ventricular septum is an anatomical structure that separates the lower chambers (ventricles) of the heart from one another. |
| ventricular trabecula myocardium morphogenesis | The process in which the anatomical structures of the trabecular cardiac ventricle muscle are generated and organized. |
5 homologous proteins in AiPD
| UniProt AC | Gene Name | Protein Name | Species | Evidence Code |
|---|---|---|---|---|
| Q90788 | NKX-2.5 | Homeobox protein Nkx-2.5 | Gallus gallus (Chicken) | PR |
| Q99801 | NKX3-1 | Homeobox protein Nkx-3.1 | Homo sapiens (Human) | PR |
| P97503 | Nkx3-2 | Homeobox protein Nkx-3.2 | Mus musculus (Mouse) | PR |
| P42582 | Nkx2-5 | Homeobox protein Nkx-2.5 | Mus musculus (Mouse) | PR |
| P46603 | HAT9 | Homeobox-leucine zipper protein HAT9 | Arabidopsis thaliana (Mouse-ear cress) | PR |
| 10 | 20 | 30 | 40 | 50 | 60 |
| MFPSPALTPT | PFSVKDILNL | EQQQRSLAAA | GELSARLEAT | LAPSSCMLAA | FKPEAYAGPE |
| 70 | 80 | 90 | 100 | 110 | 120 |
| AAAPGLPELR | AELGRAPSPA | KCASAFPAAP | AFYPRAYSDP | DPAKDPRAEK | KELCALQKAV |
| 130 | 140 | 150 | 160 | 170 | 180 |
| ELEKTEADNA | ERPRARRRRK | PRVLFSQAQV | YELERRFKQQ | RYLSAPERDQ | LASVLKLTST |
| 190 | 200 | 210 | 220 | 230 | 240 |
| QVKIWFQNRR | YKCKRQRQDQ | TLELVGLPPP | PPPPARRIAV | PVLVRDGKPC | LGDSAPYAPA |
| 250 | 260 | 270 | 280 | 290 | 300 |
| YGVGLNPYGY | NAYPAYPGYG | GAACSPGYSC | TAAYPAGPSP | AQPATAAANN | NFVNFGVGDL |
| 310 | 320 | ||||
| NAVQSPGIPQ | SNSGVSTLHG | IRAW |