P52785
Gene name |
Gucy2e (Guc2e) |
Protein name |
Retinal guanylyl cyclase 1 |
Names |
Guanylate cyclase 2E, Guanylyl cyclase GC-E |
Species |
Mus musculus (Mouse) |
KEGG Pathway |
mmu:14919 |
EC number |
4.6.1.2: Phosphorus-oxygen lyases |
Protein Class |
|
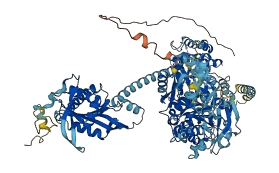
Descriptions
The autoinhibited protein was predicted that may have potential autoinhibitory elements via cis-regPred.
Autoinhibitory domains (AIDs)
Target domain |
|
Relief mechanism |
|
Assay |
cis-regPred |
Accessory elements
No accessory elements
Autoinhibited structure

Activated structure

1 structures for P52785
| Entry ID | Method | Resolution | Chain | Position | Source |
|---|---|---|---|---|---|
| AF-P52785-F1 | Predicted | AlphaFoldDB |
54 variants for P52785
| Variant ID(s) | Position | Change | Description | Diseaes Association | Provenance |
|---|---|---|---|---|---|
| rs26889949 | 15 | G>R | No | EVA | |
| rs234405523 | 19 | P>S | No | EVA | |
| rs215178643 | 21 | R>Q | No | EVA | |
| rs3389170301 | 37 | G>E | No | EVA | |
| rs3389176374 | 64 | P>S | No | EVA | |
| rs3389140432 | 72 | A>G | No | EVA | |
| rs3389173865 | 72 | A>T | No | EVA | |
| rs3389163151 | 129 | G>D | No | EVA | |
| rs3389176338 | 160 | A>E | No | EVA | |
| rs3389173844 | 173 | D>G | No | EVA | |
| rs3389170085 | 210 | A>G | No | EVA | |
| rs266246811 | 217 | L>P | No | EVA | |
| rs3389157376 | 246 | I>N | No | EVA | |
| rs3389106947 | 286 | Y>N | No | EVA | |
| rs3401150866 | 306 | R>W | No | EVA | |
| rs3389168761 | 347 | S>T | No | EVA | |
| rs3389170021 | 356 | A>D | No | EVA | |
| rs3389183066 | 370 | A>V | No | EVA | |
| rs3389106883 | 380 | S>Y | No | EVA | |
| rs26889967 | 383 | R>H | No | EVA | |
| rs3389133689 | 444 | P>S | No | EVA | |
| rs3389140440 | 453 | W>* | No | EVA | |
| rs3402141438 | 517 | H>L | No | EVA | |
| rs3402366964 | 522 | S>K | No | EVA | |
| rs3389133656 | 547 | S>N | No | EVA | |
| rs3389157355 | 549 | P>A | No | EVA | |
| rs3389106903 | 553 | T>I | No | EVA | |
| rs3389145894 | 558 | Y>H | No | EVA | |
| rs3389166914 | 564 | W>G | No | EVA | |
| rs3389168735 | 573 | H>N | No | EVA | |
| rs216124237 | 613 | P>R | No | EVA | |
| rs238685734 | 613 | P>S | No | EVA | |
| rs3389173815 | 618 | L>S | No | EVA | |
| rs3389163115 | 671 | K>R | No | EVA | |
| rs3389170332 | 688 | H>R | No | EVA | |
| rs3389180784 | 711 | W>* | No | EVA | |
| rs3389169850 | 755 | T>S | No | EVA | |
| rs1134583066 | 777 | D>N | No | EVA | |
| rs3401772450 | 828 | E>Q | No | EVA | |
| rs3389173802 | 842 | T>A | No | EVA | |
| rs3389168742 | 857 | T>A | No | EVA | |
| rs3389170002 | 891 | G>D | No | EVA | |
| rs3389180707 | 899 | S>R | No | EVA | |
| rs3389180706 | 974 | V>L | No | EVA | |
| rs3389157341 | 1002 | F>Y | No | EVA | |
| rs3389157323 | 1004 | D>N | No | EVA | |
| rs3389180718 | 1031 | L>H | No | EVA | |
| rs50451363 | 1033 | A>S | No | EVA | |
| rs3389170086 | 1034 | L>M | No | EVA | |
| rs3389140370 | 1051 | K>N | No | EVA | |
| rs3389168823 | 1058 | W>L | No | EVA | |
| rs224067952 | 1066 | N>S | No | EVA | |
| rs3389169811 | 1083 | G>S | No | EVA | |
| rs3389157335 | 1106 | T>I | No | EVA |
No associated diseases with P52785
6 regional properties for P52785
| Type | Name | Position | InterPro Accession |
|---|---|---|---|
| domain | Protein kinase domain | 471 - 811 | IPR000719 |
| domain | Adenylyl cyclase class-3/4/guanylyl cyclase | 847 - 1060 | IPR001054 |
| domain | Serine-threonine/tyrosine-protein kinase, catalytic domain | 579 - 802 | IPR001245 |
| domain | Receptor, ligand binding region | 76 - 396 | IPR001828 |
| domain | Haem NO binding associated | 823 - 868 | IPR011645 |
| conserved_site | Adenylyl cyclase class-4/guanylyl cyclase, conserved site | 990 - 1013 | IPR018297 |
Functions
| Description | ||
|---|---|---|
| EC Number | 4.6.1.2 | Phosphorus-oxygen lyases |
| Subcellular Localization |
|
|
| PANTHER Family | ||
| PANTHER Subfamily | ||
| PANTHER Protein Class | ||
| PANTHER Pathway Category | No pathway information available | |
5 GO annotations of cellular component
| Name | Definition |
|---|---|
| endoplasmic reticulum membrane | The lipid bilayer surrounding the endoplasmic reticulum. |
| integral component of membrane | The component of a membrane consisting of the gene products and protein complexes having at least some part of their peptide sequence embedded in the hydrophobic region of the membrane. |
| photoreceptor outer segment | The outer segment of a vertebrate photoreceptor that contains a stack of membrane discs embedded with photoreceptor proteins. |
| photoreceptor outer segment membrane | The membrane surrounding the outer segment of a vertebrate photoreceptor. |
| plasma membrane | The membrane surrounding a cell that separates the cell from its external environment. It consists of a phospholipid bilayer and associated proteins. |
8 GO annotations of molecular function
| Name | Definition |
|---|---|
| ATP binding | Binding to ATP, adenosine 5'-triphosphate, a universally important coenzyme and enzyme regulator. |
| GTP binding | Binding to GTP, guanosine triphosphate. |
| guanylate cyclase activity | Catalysis of the reaction: GTP = 3',5'-cyclic GMP + diphosphate. |
| identical protein binding | Binding to an identical protein or proteins. |
| peptide receptor activity | Combining with an extracellular or intracellular peptide to initiate a change in cell activity. |
| protein homodimerization activity | Binding to an identical protein to form a homodimer. |
| protein kinase activity | Catalysis of the phosphorylation of an amino acid residue in a protein, usually according to the reaction: a protein + ATP = a phosphoprotein + ADP. |
| protein-containing complex binding | Binding to a macromolecular complex. |
6 GO annotations of biological process
| Name | Definition |
|---|---|
| cGMP biosynthetic process | The chemical reactions and pathways resulting in the formation of cyclic GMP, guanosine 3',5'-phosphate. |
| cGMP-mediated signaling | Any intracellular signal transduction in which the signal is passed on within the cell via cyclic GMP (cGMP). Includes production of cGMP, and downstream effectors that further transmit the signal within the cell. |
| protein phosphorylation | The process of introducing a phosphate group on to a protein. |
| receptor guanylyl cyclase signaling pathway | The series of molecular signals initiated by an extracellular ligand binding to a receptor on the surface of the target cell where the receptor possesses guanylyl cyclase activity, and ending with the regulation of a downstream cellular process, e.g. transcription. |
| signal transduction | The cellular process in which a signal is conveyed to trigger a change in the activity or state of a cell. Signal transduction begins with reception of a signal (e.g. a ligand binding to a receptor or receptor activation by a stimulus such as light), or for signal transduction in the absence of ligand, signal-withdrawal or the activity of a constitutively active receptor. Signal transduction ends with regulation of a downstream cellular process, e.g. regulation of transcription or regulation of a metabolic process. Signal transduction covers signaling from receptors located on the surface of the cell and signaling via molecules located within the cell. For signaling between cells, signal transduction is restricted to events at and within the receiving cell. |
| visual perception | The series of events required for an organism to receive a visual stimulus, convert it to a molecular signal, and recognize and characterize the signal. Visual stimuli are detected in the form of photons and are processed to form an image. |
17 homologous proteins in AiPD
| UniProt AC | Gene Name | Protein Name | Species | Evidence Code |
|---|---|---|---|---|
| P46197 | NPR2 | Atrial natriuretic peptide receptor 2 | Bos taurus (Bovine) | PR |
| O02740 | GUCY2F | Retinal guanylyl cyclase 2 | Bos taurus (Bovine) | PR |
| P51841 | GUCY2F | Retinal guanylyl cyclase 2 | Homo sapiens (Human) | PR |
| P20594 | NPR2 | Atrial natriuretic peptide receptor 2 | Homo sapiens (Human) | PR |
| Q02846 | GUCY2D | Retinal guanylyl cyclase 1 | Homo sapiens (Human) | PR |
| Q5SDA5 | Gucy2f | Retinal guanylyl cyclase 2 | Mus musculus (Mouse) | PR |
| Q6VVW5 | Npr2 | Atrial natriuretic peptide receptor 2 | Mus musculus (Mouse) | PR |
| P18910 | Npr1 | Atrial natriuretic peptide receptor 1 | Rattus norvegicus (Rat) | PR |
| P16067 | Npr2 | Atrial natriuretic peptide receptor 2 | Rattus norvegicus (Rat) | PR |
| P51842 | Gucy2f | Retinal guanylyl cyclase 2 | Rattus norvegicus (Rat) | PR |
| P51840 | Gucy2e | Retinal guanylyl cyclase 1 | Rattus norvegicus (Rat) | PR |
| Q09435 | gcy-1 | Receptor-type guanylate cyclase gcy-1 | Caenorhabditis elegans | PR |
| O16544 | gcy-19 | Receptor-type guanylate cyclase gcy-19 | Caenorhabditis elegans | PR |
| Q18331 | gcy-11 | Receptor-type guanylate cyclase gcy-11 | Caenorhabditis elegans | PR |
| Q10029 | gcy-2 | Receptor-type guanylate cyclase gcy-2 | Caenorhabditis elegans | PR |
| X5M8U1 | gcy-17 | Receptor-type guanylate cyclase gcy-17 | Caenorhabditis elegans | PR |
| Q23682 | gcy-5 | Receptor-type guanylate cyclase gcy-5 | Caenorhabditis elegans | PR |
| 10 | 20 | 30 | 40 | 50 | 60 |
| MSAWLLPAGG | LPGAGFCVPA | RQSPSSFSRV | LRWPRPGLPG | LLLLLLLPSP | SALSAVFKVG |
| 70 | 80 | 90 | 100 | 110 | 120 |
| VLGPWACDPI | FARARPDLAA | RLAANRLNRD | FALDGGPRFE | VALLPEPCLT | PGSLGAVSSA |
| 130 | 140 | 150 | 160 | 170 | 180 |
| LSRVSGLVGP | VNPAACRPAE | LLAQEAGVAL | VPWGCPGTRA | AGTTAPAVTP | AADALYVLLR |
| 190 | 200 | 210 | 220 | 230 | 240 |
| AFRWARVALI | TAPQDLWVEA | GRALSTALRA | RGLPVALVTS | METSDRSGAR | EALGRIRDGP |
| 250 | 260 | 270 | 280 | 290 | 300 |
| RVRVVIMVMH | SVLLGGEEQR | YLLEAAEELA | LTDGSLVFLP | FDTLHYALSP | GPEALAAFVN |
| 310 | 320 | 330 | 340 | 350 | 360 |
| SSQLRRAHDA | VLTLTRRCPP | GGSVQDSLRR | AQEHQELPLD | LNLKQVSPLF | GTIYDAVFLL |
| 370 | 380 | 390 | 400 | 410 | 420 |
| AGGVKRARTA | VGGGWVSGAS | VARQVREAQV | SGFCGVLGRT | EEPSFVLLDT | DASGEQLFAT |
| 430 | 440 | 450 | 460 | 470 | 480 |
| HLLDPVLGSL | RSAGTPMHFP | RGGPAPGPDP | SCWFDPDVIC | NGGVEPGLVF | VGFLLVIGMG |
| 490 | 500 | 510 | 520 | 530 | 540 |
| LTGAFLAHYL | RHRLLHMQMA | SGPNKIILTL | EDVTFLHPPG | GSSRKVVQGS | RSSLATRSAS |
| 550 | 560 | 570 | 580 | 590 | 600 |
| DIRSVPSQPQ | ESTNVGLYEG | DWVWLKKFPG | EHHMAIRPAT | KTAFSKLREL | RHENVALYLG |
| 610 | 620 | 630 | 640 | 650 | 660 |
| LFLAGTADSP | ATPGEGILAV | VSEHCARGSL | HDLLAQREIK | LDWMFKSSLL | LDLIKGMRYL |
| 670 | 680 | 690 | 700 | 710 | 720 |
| HHRGVAHGRL | KSRNCVVDGR | FVLKVTDHGH | GRLLEAQRVL | PEPPSAEDQL | WTAPELLRDP |
| 730 | 740 | 750 | 760 | 770 | 780 |
| SLERRGTLAG | DVFSLAIIMQ | EVVCRSTPYA | MLELTPEEVI | QRVRSPPPLC | RPLVSMDQAP |
| 790 | 800 | 810 | 820 | 830 | 840 |
| MECIQLMTQC | WAEHPELRPS | MDLTFDLFKS | INKGRKTNII | DSMLRMLEQY | SSNLEDLIRE |
| 850 | 860 | 870 | 880 | 890 | 900 |
| RTEELEQEKQ | KTDRLLTQML | PPSVAEALKM | GTSVEPEYFE | EVTLYFSDIV | GFTTISAMSE |
| 910 | 920 | 930 | 940 | 950 | 960 |
| PIEVVDLLND | LYTLFDAIIG | AHDVYKVETI | GDAYMVASGL | PQRNGQRHAA | EIANMSLDIL |
| 970 | 980 | 990 | 1000 | 1010 | 1020 |
| SAVGSFRMRH | MPEVPVRIRI | GLHSGPCVAG | VVGLTMPRYC | LFGDTVNTAS | RMESTGLPYR |
| 1030 | 1040 | 1050 | 1060 | 1070 | 1080 |
| IHVNMSTVRI | LRALDQGFQM | ECRGRTELKG | KGIEDTYWLV | GRLGFNKPIP | KPPDLQPGAS |
| 1090 | 1100 | ||||
| NHGISLQEIP | PERRKKLEKA | RPGQFTGK |