P48455
Gene name |
Ppp3cc (Calnc) |
Protein name |
Serine/threonine-protein phosphatase 2B catalytic subunit gamma isoform |
Names |
CAM-PRP catalytic subunit, Calcineurin, testis-specific catalytic subunit, Calmodulin-dependent calcineurin A subunit gamma isoform |
Species |
Mus musculus (Mouse) |
KEGG Pathway |
mmu:19057 |
EC number |
3.1.3.16: Phosphoric monoester hydrolases |
Protein Class |
|
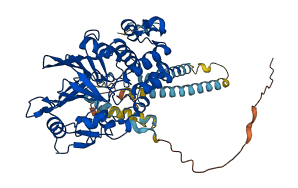
Descriptions
(Annotation based on sequence homology with P63328)
Calcium-dependent, calmodulin-stimulated protein phosphatase calcineurin (CN) plays a role in the transduction of intracellular Ca2+-dependent signals. CN is a heterodimer composed of a catalytic subunit A (CNA) and an essential regulatory subunit B (CNB).
CNA contains two autoinhibitory regions, which interfere with the function of the catalytic domain: autoinhibitory segment (AIS) and autoinhibitory domain (AID). The AIS interacts with a hydrophobic intersubunit groove formed at the junction of CNA and CNB, and disruption of the AIS interaction results in partial stimulation of CN activity. In addition, the binding partner calmodulin regulates the orientation of AID with respect to the catalytic core, resulting in incomplete activation of CN.
Autoinhibitory domains (AIDs)
Target domain |
|
Relief mechanism |
|
Assay |
cis-regPred |
Accessory elements
No accessory elements
Autoinhibited structure

Activated structure

1 structures for P48455
| Entry ID | Method | Resolution | Chain | Position | Source |
|---|---|---|---|---|---|
| AF-P48455-F1 | Predicted | AlphaFoldDB |
27 variants for P48455
| Variant ID(s) | Position | Change | Description | Diseaes Association | Provenance |
|---|---|---|---|---|---|
| rs3389312834 | 41 | L>I | No | EVA | |
| rs3389351559 | 55 | E>G | No | EVA | |
| rs3389343600 | 68 | L>M | No | EVA | |
| rs13463775 | 99 | E>G | No | EVA | |
| rs3389329989 | 140 | L>V | No | EVA | |
| rs3389330063 | 149 | C>S | No | EVA | |
| rs3389348329 | 166 | Y>C | No | EVA | |
| rs3389348383 | 170 | V>M | No | EVA | |
| rs3389338222 | 193 | C>Y | No | EVA | |
| rs3389343596 | 204 | C>* | No | EVA | |
| rs3404479796 | 227 | L>P | No | EVA | |
| rs3405335154 | 228 | W>* | No | EVA | |
| rs3404479773 | 235 | Y>* | No | EVA | |
| rs3405246670 | 243 | H>Q | No | EVA | |
| rs3389338317 | 265 | L>* | No | EVA | |
| rs3389329995 | 275 | R>I | No | EVA | |
| rs3389336339 | 282 | A>V | No | EVA | |
| rs1133277860 | 300 | T>M | No | EVA | |
| rs250940036 | 374 | N>S | No | EVA | |
| rs3404545559 | 377 | D>E | No | EVA | |
| rs3405012218 | 378 | E>* | No | EVA | |
| rs3405547204 | 402 | V>I | No | EVA | |
| rs3404479762 | 446 | E>P | No | EVA | |
| rs219281028 | 447 | E>D | No | EVA | |
| rs3389323677 | 485 | H>R | No | EVA | |
| rs3389336305 | 487 | D>E | No | EVA | |
| rs260023365 | 500 | P>S | No | EVA |
No associated diseases with P48455
5 regional properties for P48455
| Type | Name | Position | InterPro Accession |
|---|---|---|---|
| domain | Protein kinase domain | 447 - 726 | IPR000719 |
| active_site | Serine/threonine-protein kinase, active site | 569 - 581 | IPR008271 |
| domain | Wall-associated receptor kinase | 202 - 310 | IPR013695 |
| conserved_site | EGF-like calcium-binding, conserved site | 338 - 365 | IPR018097 |
| domain | Wall-associated receptor kinase, galacturonan-binding domain | 31 - 83 | IPR025287 |
Functions
| Description | ||
|---|---|---|
| EC Number | 3.1.3.16 | Phosphoric monoester hydrolases |
| Subcellular Localization |
|
|
| PANTHER Family | ||
| PANTHER Subfamily | ||
| PANTHER Protein Class | ||
| PANTHER Pathway Category | No pathway information available | |
11 GO annotations of cellular component
| Name | Definition |
|---|---|
| calcineurin complex | A heterodimeric calcium ion and calmodulin dependent protein phosphatase composed of catalytic and regulatory subunits; the regulatory subunit is very similar in sequence to calmodulin. |
| cytoplasm | The contents of a cell excluding the plasma membrane and nucleus, but including other subcellular structures. |
| cytosol | The part of the cytoplasm that does not contain organelles but which does contain other particulate matter, such as protein complexes. |
| glutamatergic synapse | A synapse that uses glutamate as a neurotransmitter. |
| mitochondrion | A semiautonomous, self replicating organelle that occurs in varying numbers, shapes, and sizes in the cytoplasm of virtually all eukaryotic cells. It is notably the site of tissue respiration. |
| presynapse | The part of a synapse that is part of the presynaptic cell. |
| presynaptic cytosol | The region of the cytosol consisting of all cytosol that is part of the presynapse. |
| protein serine/threonine phosphatase complex | A complex, normally consisting of a catalytic and a regulatory subunit, which catalyzes the removal of a phosphate group from a serine or threonine residue of a protein. |
| sperm flagellum | A microtubule-based flagellum (or cilium) that is part of a sperm, a mature male germ cell that develops from a spermatid. |
| sperm midpiece | The highly organized segment of the sperm flagellum which begins at the connecting piece and is characterized by the presence of 9 outer dense fibers (ODFs) that lie outside each of the 9 outer axonemal microtubule doublets and by a sheath of mitochondria that encloses the ODFs and the axoneme; the midpiece terminates about one-fourth of the way down the sperm flagellum at the annulus, which marks the beginning of the principal piece. |
| sperm mitochondrial sheath | The tightly packed helical sheath of ATP-producing mitochondria restricted to the midpiece of the sperm flagellum. |
5 GO annotations of molecular function
| Name | Definition |
|---|---|
| calcium-dependent protein serine/threonine phosphatase activity | Catalysis of the reactions: protein serine phosphate + H2O = protein serine + phosphate; and protein threonine phosphate + H2O = protein threonine + phosphate. These reactions require the presence of calcium ions. |
| calmodulin binding | Binding to calmodulin, a calcium-binding protein with many roles, both in the calcium-bound and calcium-free states. |
| calmodulin-dependent protein phosphatase activity | Catalysis of the reaction: protein serine/threonine phosphate + H2O = protein serine/threonine + phosphate, dependent on the presence of calcium-bound calmodulin. |
| metal ion binding | Binding to a metal ion. |
| myosin phosphatase activity | Catalysis of the reaction: phosphomyosin + H2O = myosin + phosphate. |
11 GO annotations of biological process
| Name | Definition |
|---|---|
| calcineurin-mediated signaling | Any intracellular signal transduction in which the signal is passed on within the cell by activation of a transcription factor as a consequence of dephosphorylation by Ca(2+)-activated calcineurin. The process begins with calcium-dependent activation of the phosphatase calcineurin. Calcineurin is a calcium- and calmodulin-dependent serine/threonine protein phosphatase with a conserved function in eukaryotic species from yeast to humans. In yeast and fungi, calcineurin regulates stress signaling and cell cycle, and sporulation and virulence in pathogenic fungi. In metazoans, calcineurin is involved in cell commitment, organogenesis and organ development and immune function of T-lymphocytes. By a conserved mechanism, calcineurin phosphatase activates fungal Crz1 and mammalian NFATc by dephosphorylation and translocation of these transcription factors to the nucleus to regulate gene expression. |
| calcineurin-NFAT signaling cascade | Any intracellular signal transduction in which the signal is passed on within the cell by activation of a member of the NFAT protein family as a consequence of NFAT dephosphorylation by Ca(2+)-activated calcineurin. The cascade begins with calcium-dependent activation of the phosphatase calcineurin. Calcineurin dephosphorylates multiple phosphoserine residues on NFAT, resulting in the translocation of NFAT to the nucleus. The cascade ends with regulation of transcription by NFAT. The calcineurin-NFAT cascade lies downstream of many cell surface receptors, including G protein-coupled receptors (GPCRs) and receptor tyrosine kinases (RTKs) that signal to mobilize calcium ions (Ca2+). |
| negative regulation of calcium ion import across plasma membrane | Any process that stops, prevents or reduces the frequency, rate or extent of calcium ion import across plasma membrane. |
| negative regulation of voltage-gated calcium channel activity | Any process that stops, prevents or reduces the frequency, rate or extent of voltage-gated calcium channel activity. |
| penetration of zona pellucida | The infiltration by sperm of the zona pellucida to reach the oocyte. The process involves digestive enzymes from a modified lysosome called the acrosome, situated at the head of the sperm. |
| positive regulation of calcineurin-NFAT signaling cascade | Any process that activates or increases the frequency, rate or extent of signaling via the calcineurin-NFAT signaling cascade. |
| positive regulation of calcium ion import across plasma membrane | Any process that activates or increases the frequency, rate or extent of calcium ion import across plasma membrane. |
| positive regulation of synaptic vesicle endocytosis | Any process that activates or increases the frequency, rate or extent of synaptic vesicle endocytosis. |
| positive regulation of voltage-gated calcium channel activity | Any process that activates or increases the frequency, rate or extent of voltage-gated calcium channel activity. |
| protein dephosphorylation | The process of removing one or more phosphoric residues from a protein. |
| regulation of synaptic vesicle endocytosis | Any process that modulates the frequency, rate or extent of synaptic vesicle endocytosis. |
13 homologous proteins in AiPD
| UniProt AC | Gene Name | Protein Name | Species | Evidence Code |
|---|---|---|---|---|
| P23287 | CNA1 | Serine/threonine-protein phosphatase 2B catalytic subunit A1 | Saccharomyces cerevisiae (strain ATCC 204508 / S288c) (Baker's yeast) | PR |
| P48452 | PPP3CA | Protein phosphatase 3 catalytic subunit alpha | Bos taurus (Bovine) | SS |
| P48456 | CanA1 | Serine/threonine-protein phosphatase 2B catalytic subunit 1 | Drosophila melanogaster (Fruit fly) | SS |
| Q27889 | Pp2B-14D | Serine/threonine-protein phosphatase 2B catalytic subunit 2 | Drosophila melanogaster (Fruit fly) | SS |
| Q9VXF1 | CanA-14F | Serine/threonine-protein phosphatase 2B catalytic subunit 3 | Drosophila melanogaster (Fruit fly) | SS |
| P16298 | PPP3CB | Serine/threonine-protein phosphatase 2B catalytic subunit beta isoform | Homo sapiens (Human) | EV |
| Q08209 | PPP3CA | Serine/threonine-protein phosphatase 2B catalytic subunit alpha isoform | Homo sapiens (Human) | EV |
| P48454 | PPP3CC | Serine/threonine-protein phosphatase 2B catalytic subunit gamma isoform | Homo sapiens (Human) | SS |
| P48453 | Ppp3cb | Serine/threonine-protein phosphatase 2B catalytic subunit beta isoform | Mus musculus (Mouse) | SS |
| P63328 | Ppp3ca | Serine/threonine-protein phosphatase 2B catalytic subunit alpha isoform | Mus musculus (Mouse) | EV |
| P20651 | Ppp3cb | Serine/threonine-protein phosphatase 2B catalytic subunit beta isoform | Rattus norvegicus (Rat) | SS |
| P63329 | Ppp3ca | Serine/threonine-protein phosphatase 2B catalytic subunit alpha isoform | Rattus norvegicus (Rat) | SS |
| Q0G819 | tax-6 | Serine/threonine-protein phosphatase 2B catalytic subunit | Caenorhabditis elegans | SS |
| 10 | 20 | 30 | 40 | 50 | 60 |
| MSVRRPQFST | TERVIKAVPF | PPTRRLTLKE | VFENGKPKMD | LLKNHLVKEG | RVEEEVALKI |
| 70 | 80 | 90 | 100 | 110 | 120 |
| INDGAAILKQ | EKTMIEVEAP | ITVCGDVHGQ | FFDLMKLFEV | GGSPSNTRYL | FLGDYVDRGY |
| 130 | 140 | 150 | 160 | 170 | 180 |
| FSIECVLYLW | SLKINHPKTL | FLLRGNHECR | HLTEYFTFKQ | ECRIKYSEMV | YDACMHTFDC |
| 190 | 200 | 210 | 220 | 230 | 240 |
| LPLAALLNQQ | FLCVHGGMSP | EITCLEDIRK | LDRFSEPPAF | GPVCDLLWSD | PLEDYGSEKT |
| 250 | 260 | 270 | 280 | 290 | 300 |
| LEHYTHNTVR | GCSYFFSYPA | VCEFLQNNSL | LSIIRAHEAQ | DAGYRMYRKN | QATGFPSLIT |
| 310 | 320 | 330 | 340 | 350 | 360 |
| IFSAPNYLDV | YNNKAAVLKY | ENNVMNIRQF | NCSPHPYWLP | NFMDVFTWSL | PFVGEKVTEM |
| 370 | 380 | 390 | 400 | 410 | 420 |
| LVNILNICSD | EEMNVTDEEG | ATTGRKEVIK | NKIRAIGKMA | RVFTVLREES | ENVLTLKGLT |
| 430 | 440 | 450 | 460 | 470 | 480 |
| PTGTLPLGVL | SGGKQTIETA | KQEAAEEREA | IRGFTIAHRI | RSFEEARGLD | RINERMPPRK |
| 490 | 500 | 510 | |||
| EASYHHDAGR | MHSHSHPPHP | QASRRTDHGK | KAL |