P27870
Gene name |
Vav1 (Vav) |
Protein name |
Proto-oncogene vav |
Names |
p95vav |
Species |
Mus musculus (Mouse) |
KEGG Pathway |
mmu:22324 |
EC number |
|
Protein Class |
PROTEIN VAV (PTHR45818) |
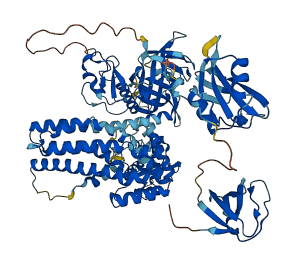
Descriptions
The Vav family is a group of tyrosine phosphorylation-dependent guanine nucleotide exchange factors (GEFs) that activate members of the Rac and Rho families of guanosine triphosphatases (GTPases) downstream of protein tyrosine kinases. When the tyrosine residues within the acidic (Ac) region are non-phosphorylated, the CH domain and Ac region occlude the GTPase-binding site within DH domain. The interaction is stabilized by the interaction between the hydroxyl group of Tyr174 within Ac region and GTPase binding site as well as the interaction between CH domain and DH-PH domains. The Y174F mutant displays constitutive and phosphorylation-independent catalytic activity. In addition to the N-terminal autoinhibitory regions, the truncation of C-terminal SH3 domain also relives the autoinhibition. C-terminal SH3 domain interacts with DH-PH domains and the intramolecular interaction occludes the GTPase-binding site of DH domain.
Autoinhibitory domains (AIDs)
Target domain |
194-373 (DH domain) |
Relief mechanism |
PTM |
Assay |
Deletion assay, Structural analysis |
Target domain |
194-373 (DH domain) |
Relief mechanism |
PTM |
Assay |
Mutagenesis experiment, Deletion assay, Structural analysis |
Target domain |
194-373 (DH domain) |
Relief mechanism |
Partner binding, PTM |
Assay |
Deletion assay, Structural analysis |
Accessory elements
No accessory elements
References
- Barreira M et al. (2014) "The C-terminal SH3 domain contributes to the intramolecular inhibition of Vav family proteins", Science signaling, 7, ra35
- Rapley J et al. (2008) "Crucial structural role for the PH and C1 domains of the Vav1 exchange factor", EMBO reports, 9, 655-61
- Aghazadeh B et al. (2000) "Structural basis for relief of autoinhibition of the Dbl homology domain of proto-oncogene Vav by tyrosine phosphorylation", Cell, 102, 625-33
Autoinhibited structure
Activated structure

7 variants for P27870
| Variant ID(s) | Position | Change | Description | Diseaes Association | Provenance |
|---|---|---|---|---|---|
| rs49416177 | 29 | Q>H | No | Ensembl | |
| rs51086382 | 32 | E>D | No | Ensembl | |
| rs51833044 | 53 | Q>H | No | Ensembl | |
| rs46309023 | 54 | A>G | No | Ensembl | |
| rs48127552 | 65 | Q>H | No | Ensembl | |
| rs1131965516 | 153 | A>P | No | Ensembl | |
| rs237938200 | 305 | Q>H | No | Ensembl |
No associated diseases with P27870
11 regional properties for P27870
| Type | Name | Position | InterPro Accession |
|---|---|---|---|
| domain | Dbl homology (DH) domain | 194 - 373 | IPR000219 |
| conserved_site | Guanine-nucleotide dissociation stimulator, CDC24, conserved site | 322 - 347 | IPR001331 |
| domain | SH3 domain | 592 - 660 | IPR001452-1 |
| domain | SH3 domain | 782 - 842 | IPR001452-2 |
| domain | Calponin homology domain | 1 - 119 | IPR001715 |
| domain | Pleckstrin homology domain | 402 - 506 | IPR001849 |
| domain | Calmodulin-regulated spectrin-associated protein-like, Calponin-homology domain | 19 - 102 | IPR022613 |
| domain | VAV1 protein, second SH3 domain | 786 - 839 | IPR035729 |
| domain | VAV1 protein, first SH3 domain | 596 - 658 | IPR035730 |
| domain | VAV1, SH2 domain | 665 - 767 | IPR035879 |
| domain | Vav, PH domain | 385 - 508 | IPR037832 |
Functions
| Description | ||
|---|---|---|
| EC Number | ||
| Subcellular Localization |
|
|
| PANTHER Family | PTHR45818 | PROTEIN VAV |
| PANTHER Subfamily | PTHR45818:SF2 | PROTO-ONCOGENE VAV |
| PANTHER Protein Class | ||
| PANTHER Pathway Category |
B cell activation vav T cell activation vav PDGF signaling pathway Vav Inflammation mediated by chemokine and cytokine signaling pathway GEF |
|
2 GO annotations of cellular component
| Name | Definition |
|---|---|
| cell-cell junction | A cell junction that forms a connection between two or more cells of an organism; excludes direct cytoplasmic intercellular bridges, such as ring canals in insects. |
| cytosol | The part of the cytoplasm that does not contain organelles but which does contain other particulate matter, such as protein complexes. |
4 GO annotations of molecular function
| Name | Definition |
|---|---|
| guanyl-nucleotide exchange factor activity | Stimulates the exchange of GDP to GTP on a signaling GTPase, changing its conformation to its active form. Guanine nucleotide exchange factors (GEFs) act by stimulating the release of guanosine diphosphate (GDP) to allow binding of guanosine triphosphate (GTP), which is more abundant in the cell under normal cellular physiological conditions. |
| metal ion binding | Binding to a metal ion. |
| phosphorylation-dependent protein binding | Binding to a protein upon phosphorylation of the target protein. |
| phosphotyrosine residue binding | Binding to a phosphorylated tyrosine residue within a protein. |
15 GO annotations of biological process
| Name | Definition |
|---|---|
| G protein-coupled receptor signaling pathway | The series of molecular signals initiated by a ligand binding to its receptor, in which the activated receptor promotes the exchange of GDP for GTP on the alpha-subunit of an associated heterotrimeric G-protein complex. The GTP-bound activated alpha-G-protein then dissociates from the beta- and gamma-subunits to further transmit the signal within the cell. The pathway begins with receptor-ligand interaction, and ends with regulation of a downstream cellular process. The pathway can start from the plasma membrane, Golgi or nuclear membrane. |
| immune response | Any immune system process that functions in the calibrated response of an organism to a potential internal or invasive threat. |
| integrin-mediated signaling pathway | The series of molecular signals initiated by an extracellular ligand binding to an integrin on the surface of a target cell, and ending with the regulation of a downstream cellular process, e.g. transcription. |
| intracellular signal transduction | The process in which a signal is passed on to downstream components within the cell, which become activated themselves to further propagate the signal and finally trigger a change in the function or state of the cell. |
| neutrophil chemotaxis | The directed movement of a neutrophil cell, the most numerous polymorphonuclear leukocyte found in the blood, in response to an external stimulus, usually an infection or wounding. |
| phagocytosis | A vesicle-mediated transport process that results in the engulfment of external particulate material by phagocytes and their delivery to the lysosome. The particles are initially contained within phagocytic vacuoles (phagosomes), which then fuse with primary lysosomes to effect digestion of the particles. |
| positive regulation of cell adhesion | Any process that activates or increases the frequency, rate or extent of cell adhesion. |
| positive regulation of GTPase activity | Any process that activates or increases the activity of a GTPase. |
| positive regulation of natural killer cell mediated cytotoxicity | Any process that activates or increases the frequency, rate or extent of natural killer cell mediated cytotoxicity. |
| reactive oxygen species metabolic process | The chemical reactions and pathways involving a reactive oxygen species, any molecules or ions formed by the incomplete one-electron reduction of oxygen. They contribute to the microbicidal activity of phagocytes, regulation of signal transduction and gene expression, and the oxidative damage to biopolymers. |
| regulation of cell size | Any process that modulates the size of a cell. |
| regulation of GTPase activity | Any process that modulates the rate of GTP hydrolysis by a GTPase. |
| small GTPase mediated signal transduction | The series of molecular signals in which a small monomeric GTPase relays a signal. |
| T cell activation | The change in morphology and behavior of a mature or immature T cell resulting from exposure to a mitogen, cytokine, chemokine, cellular ligand, or an antigen for which it is specific. |
| T cell differentiation | The process in which a precursor cell type acquires characteristics of a more mature T-cell. A T cell is a type of lymphocyte whose definin characteristic is the expression of a T cell receptor complex. |
9 homologous proteins in AiPD
| UniProt AC | Gene Name | Protein Name | Species | Evidence Code |
|---|---|---|---|---|
| Q08DN7 | VAV1 | Proto-oncogene vav | Bos taurus (Bovine) | SS |
| Q9NHV9 | Vav | Protein vav | Drosophila melanogaster (Fruit fly) | SS |
| P52735 | VAV2 | Guanine nucleotide exchange factor VAV2 | Homo sapiens (Human) | SS |
| Q9UKW4 | VAV3 | Guanine nucleotide exchange factor VAV3 | Homo sapiens (Human) | SS |
| P15498 | VAV1 | Proto-oncogene vav | Homo sapiens (Human) | EV SS |
| Q60992 | Vav2 | Guanine nucleotide exchange factor VAV2 | Mus musculus (Mouse) | SS |
| Q9R0C8 | Vav3 | Guanine nucleotide exchange factor VAV3 | Mus musculus (Mouse) | SS |
| P54100 | Vav1 | Proto-oncogene vav | Rattus norvegicus (Rat) | SS |
| Q45FX5 | vav-1 | Protein vav-1 | Caenorhabditis elegans | SS |
| 10 | 20 | 30 | 40 | 50 | 60 |
| MELWRQCTHW | LIQCRVLPPS | HRVTWEGAQV | CELAQALRDG | VLLCQLLNNL | LPQAINLREV |
| 70 | 80 | 90 | 100 | 110 | 120 |
| NLRPQMSQFL | CLKNIRTFLS | TCCEKFGLKR | SELFEAFDLF | DVQDFGKVIY | TLSALSWTPI |
| 130 | 140 | 150 | 160 | 170 | 180 |
| AQNKGIMPFP | TEDSALNDED | IYSGLSDQID | DTAEEDEDLY | DCVENEEAEG | DEIYEDLMRL |
| 190 | 200 | 210 | 220 | 230 | 240 |
| ESVPTPPKMT | EYDKRCCCLR | EIQQTEEKYT | DTLGSIQQHF | MKPLQRFLKP | QDMETIFVNI |
| 250 | 260 | 270 | 280 | 290 | 300 |
| EELFSVHTHF | LKELKDALAG | PGATTLYQVF | IKYKERFLVY | GRYCSQVESA | SKHLDQVATA |
| 310 | 320 | 330 | 340 | 350 | 360 |
| REDVQMKLEE | CSQRANNGRF | TLRDLLMVPM | QRVLKYHLLL | QELVKHTQDA | TEKENLRLAL |
| 370 | 380 | 390 | 400 | 410 | 420 |
| DAMRDLAQCV | NEVKRDNETL | RQITNFQLSI | ENLDQSLANY | GRPKIDGELK | ITSVERRSKT |
| 430 | 440 | 450 | 460 | 470 | 480 |
| DRYAFLLDKA | LLICKRRGDS | YDLKASVNLH | SFQVRDDSSG | ERDNKKWSHM | FLLIEDQGAQ |
| 490 | 500 | 510 | 520 | 530 | 540 |
| GYELFFKTRE | LKKKWMEQFE | MAISNIYPEN | ATANGHDFQM | FSFEETTSCK | ACQMLLRGTF |
| 550 | 560 | 570 | 580 | 590 | 600 |
| YQGYRCYRCR | APAHKECLGR | VPPCGRHGQD | FAGTMKKDKL | HRRAQDKKRN | ELGLPKMEVF |
| 610 | 620 | 630 | 640 | 650 | 660 |
| QEYYGIPPPP | GAFGPFLRLN | PGDIVELTKA | EAEHNWWEGR | NTATNEVGWF | PCNRVHPYVH |
| 670 | 680 | 690 | 700 | 710 | 720 |
| GPPQDLSVHL | WYAGPMERAG | AEGILTNRSD | GTYLVRQRVK | DTAEFAISIK | YNVEVKHIKI |
| 730 | 740 | 750 | 760 | 770 | 780 |
| MTSEGLYRIT | EKKAFRGLLE | LVEFYQQNSL | KDCFKSLDTT | LQFPYKEPER | RAISKPPAGS |
| 790 | 800 | 810 | 820 | 830 | 840 |
| TKYFGTAKAR | YDFCARDRSE | LSLKEGDIIK | ILNKKGQQGW | WRGEIYGRIG | WFPSNYVEED |
| YSEYC |