P26042
Gene name |
MSN |
Protein name |
Moesin |
Names |
Membrane-organizing extension spike protein |
Species |
Sus scrofa (Pig) |
KEGG Pathway |
ssc:494458 |
EC number |
|
Protein Class |
|
Descriptions
(Annotation based on sequence homology with P26038)
MSN is a moesin protein and belongs to the ezrin-radixin-moesin (ERM) protein family, directly involved in the cytoskeleton-membrane crosslinking. Functional N-terminal FERM domain of ERM attaches to the membrane by binding specific membrane proteins while the last 34 residues of the C-terminal tail domain bind actin filaments. The autoinhibitory domain is positions at the C-terminal tail domain of ERM, where FERM domain of ERM is bound tightly via phosphotyrosine binding (PTB), pleckstrin homology (PH), and Enabled/VASP Homology 1 (EVH1) domains, thus masking the binding sites for other molecules. ERM is activated through phosphorylation at Thr558 weakening the FERM/tail binding and, unmasks the binding sites of membrane protein and actin filaments in the presence of phospholipids.
Autoinhibitory domains (AIDs)
Target domain |
|
Relief mechanism |
|
Assay |
cis-regPred |
Accessory elements
No accessory elements
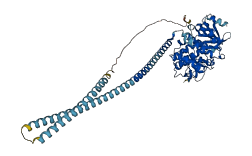
Autoinhibited structure

Activated structure

1 structures for P26042
| Entry ID | Method | Resolution | Chain | Position | Source |
|---|---|---|---|---|---|
| AF-P26042-F1 | Predicted | AlphaFoldDB |
No variants for P26042
| Variant ID(s) | Position | Change | Description | Diseaes Association | Provenance |
|---|---|---|---|---|---|
| No variants for P26042 | |||||
No associated diseases with P26042
10 regional properties for P26042
| Type | Name | Position | InterPro Accession |
|---|---|---|---|
| domain | FERM domain | 5 - 295 | IPR000299 |
| domain | Ezrin/radixin/moesin, C-terminal | 502 - 577 | IPR011259 |
| domain | FERM, N-terminal | 9 - 70 | IPR018979 |
| domain | FERM, C-terminal PH-like domain | 210 - 299 | IPR018980 |
| conserved_site | FERM conserved site | 58 - 88 | IPR019747-1 |
| conserved_site | FERM conserved site | 176 - 205 | IPR019747-2 |
| domain | FERM central domain | 91 - 206 | IPR019748 |
| domain | Band 4.1 domain | 1 - 206 | IPR019749 |
| domain | ERM family, FERM domain C-lobe | 200 - 296 | IPR041789 |
| domain | Ezrin/radixin/moesin, alpha-helical domain | 337 - 456 | IPR046810 |
Functions
9 GO annotations of cellular component
| Name | Definition |
|---|---|
| adherens junction | A cell-cell junction composed of the epithelial cadherin-catenin complex. The epithelial cadherins, or E-cadherins, of each interacting cell extend through the plasma membrane into the extracellular space and bind to each other. The E-cadherins bind to catenins on the cytoplasmic side of the membrane, where the E-cadherin-catenin complex binds to cytoskeletal components and regulatory and signaling molecules. |
| apical part of cell | The region of a polarized cell that forms a tip or is distal to a base. For example, in a polarized epithelial cell, the apical region has an exposed surface and lies opposite to the basal lamina that separates the epithelium from other tissue. |
| apical plasma membrane | The region of the plasma membrane located at the apical end of the cell. |
| cytoplasm | The contents of a cell excluding the plasma membrane and nucleus, but including other subcellular structures. |
| cytoskeleton | A cellular structure that forms the internal framework of eukaryotic and prokaryotic cells. The cytoskeleton includes intermediate filaments, microfilaments, microtubules, the microtrabecular lattice, and other structures characterized by a polymeric filamentous nature and long-range order within the cell. The various elements of the cytoskeleton not only serve in the maintenance of cellular shape but also have roles in other cellular functions, including cellular movement, cell division, endocytosis, and movement of organelles. |
| filopodium | Thin, stiff, actin-based protrusion extended by the leading edge of a motile cell such as a crawling fibroblast or amoeba, or an axonal or dendritic growth cone, or a dendritic shaft. |
| microvillus | Thin cylindrical membrane-covered projections on the surface of an animal cell containing a core bundle of actin filaments. Present in especially large numbers on the absorptive surface of intestinal cells. |
| microvillus membrane | The portion of the plasma membrane surrounding a microvillus. |
| plasma membrane | The membrane surrounding a cell that separates the cell from its external environment. It consists of a phospholipid bilayer and associated proteins. |
2 GO annotations of molecular function
| Name | Definition |
|---|---|
| actin binding | Binding to monomeric or multimeric forms of actin, including actin filaments. |
| cell adhesion molecule binding | Binding to a cell adhesion molecule. |
8 GO annotations of biological process
| Name | Definition |
|---|---|
| immunological synapse formation | The formation of an area of close contact between a lymphocyte (T-, B-, or natural killer cell) and a target cell through the clustering of particular signaling and adhesion molecules and their associated membrane rafts on both the lymphocyte and target cell, which facilitates activation of the lymphocyte, transfer of membrane from the target cell to the lymphocyte, and in some situations killing of the target cell through release of secretory granules and/or death-pathway ligand-receptor interaction. |
| positive regulation of early endosome to late endosome transport | Any process that activates or increases the frequency, rate or extent of early endosome to late endosome transport. |
| positive regulation of protein localization to early endosome | Any process that activates or increases the frequency, rate or extent of protein localization to early endosome. |
| regulation of cell shape | Any process that modulates the surface configuration of a cell. |
| regulation of organelle assembly | Any process that modulates the frequency, rate or extent of organelle assembly. |
| T cell aggregation | The adhesion of one T cell to one or more other T cells via adhesion molecules. |
| T cell migration | The movement of a T cell within or between different tissues and organs of the body. |
| T cell proliferation | The expansion of a T cell population by cell division. Follows T cell activation. |
21 homologous proteins in AiPD
| UniProt AC | Gene Name | Protein Name | Species | Evidence Code |
|---|---|---|---|---|
| Q32LP2 | RDX | Radixin | Bos taurus (Bovine) | SS |
| P31976 | EZR | Ezrin | Bos taurus (Bovine) | PR |
| Q2HJ49 | MSN | Moesin | Bos taurus (Bovine) | PR |
| Q9PU45 | RDX | Radixin | Gallus gallus (Chicken) | PR |
| Q24564 | Mer | Moesin/ezrin/radixin homolog 2 | Drosophila melanogaster (Fruit fly) | SS |
| P46150 | Moe | Moesin/ezrin/radixin homolog 1 | Drosophila melanogaster (Fruit fly) | SS |
| P35240 | NF2 | Merlin | Homo sapiens (Human) | EV |
| P15311 | EZR | Ezrin | Homo sapiens (Human) | EV |
| P35241 | RDX | Radixin | Homo sapiens (Human) | SS |
| Q3KP66 | INAVA | Innate immunity activator protein | Homo sapiens (Human) | PR |
| P26038 | MSN | Moesin | Homo sapiens (Human) | EV |
| P26043 | Rdx | Radixin | Mus musculus (Mouse) | SS |
| A2AD83 | Frmd7 | FERM domain-containing protein 7 | Mus musculus (Mouse) | PR |
| P26040 | Ezr | Ezrin | Mus musculus (Mouse) | SS |
| P46662 | Nf2 | Merlin | Mus musculus (Mouse) | SS |
| P26041 | Msn | Moesin | Mus musculus (Mouse) | SS |
| P26044 | RDX | Radixin | Sus scrofa (Pig) | SS |
| Q63648 | Nf2 | Merlin | Rattus norvegicus (Rat) | SS |
| P31977 | Ezr | Ezrin | Rattus norvegicus (Rat) | SS |
| O35763 | Msn | Moesin | Rattus norvegicus (Rat) | SS |
| Q6Q413 | nf2b | NF2, moesin-ezrin-radixin-like | Danio rerio (Zebrafish) (Brachydanio rerio) | SS |
| 10 | 20 | 30 | 40 | 50 | 60 |
| MPKTINVRVT | TMDAELEFAI | QPNTTGKQLF | DQVVKTIGLR | EVWFFGLQYQ | DTKGFSTWLK |
| 70 | 80 | 90 | 100 | 110 | 120 |
| LNKKVTAQDV | RKESPLLFKF | RAKFYPEDVS | EELIQDITQR | LFFLQVKEGI | LNDDIYCPPE |
| 130 | 140 | 150 | 160 | 170 | 180 |
| TAVLLASYAV | QSKYGDFNKE | VHKSGYLAGD | KLLPQRVLEQ | HKLNKDQWEE | RIQVWHEEHR |
| 190 | 200 | 210 | 220 | 230 | 240 |
| GMLREDAVLE | YLKIAQDLEM | YGVNYFSSKN | KKGSELWLGV | DALGLNIYEQ | NDRLTPKIGF |
| 250 | 260 | 270 | 280 | 290 | 300 |
| PWSEIRNISF | NDKKFVIKPI | DKKAPDFVFY | APRLRINKRI | LALCMGNHEL | YMRRRKPDTI |
| 310 | 320 | 330 | 340 | 350 | 360 |
| EVQQMKAQAR | EEKHQKQMER | ALLENEKKKR | EMAEKEKEKI | EREKEELMER | LKQIEEQTKK |
| 370 | 380 | 390 | 400 | 410 | 420 |
| AQQELEEQTR | RALALEQERK | RAQSEAEKLA | KERQEAEEAK | EALLKASRDQ | KKTQEQLALE |
| 430 | 440 | 450 | 460 | 470 | 480 |
| MAELTARISQ | LEMARQKKES | EAAEWQQKAQ | MVQEDLEKTR | AELKTAMSTP | HGAEPAENDQ |
| 490 | 500 | 510 | 520 | 530 | 540 |
| DEQDENGAEA | SADLRADAMA | KDRSEEERTT | EAEKNERVQK | HLKALTSELA | NARDESKKTA |
| 550 | 560 | 570 | |||
| NDMIHAENMR | LGRDKYKTLR | QIRQGNTKQR | IDEFESM |