P0CE68
Gene name |
NFT1 (YKR103W) |
Protein name |
ABC transporter NFT1 |
Names |
New full-length MRP-type transporter 1 |
Species |
Saccharomyces cerevisiae (strain ATCC 204508 / S288c) (Baker's yeast) |
KEGG Pathway |
sce:YKR103W |
EC number |
|
Protein Class |
|
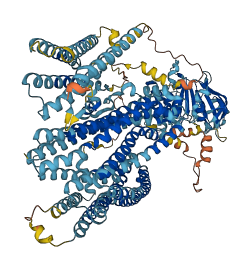
Descriptions
The autoinhibited protein was predicted that may have potential autoinhibitory elements via cis-regPred.
Autoinhibitory domains (AIDs)
Target domain |
|
Relief mechanism |
|
Assay |
cis-regPred |
Accessory elements
No accessory elements
Autoinhibited structure

Activated structure

1 structures for P0CE68
| Entry ID | Method | Resolution | Chain | Position | Source |
|---|---|---|---|---|---|
| AF-P0CE68-F1 | Predicted | AlphaFoldDB |
28 variants for P0CE68
| Variant ID(s) | Position | Change | Description | Diseaes Association | Provenance |
|---|---|---|---|---|---|
| s11-652743 | 9 | Y>F | No | SGRP | |
| s11-652871 | 52 | G>R | No | SGRP | |
| s11-653085 | 123 | S>N | No | SGRP | |
| s11-653091 | 125 | P>L | No | SGRP | |
| s11-653139 | 141 | F>Y | No | SGRP | |
| s11-653432 | 239 | N>D | No | SGRP | |
| s11-653543 | 276 | D>N | No | SGRP | |
| s11-653694 | 326 | F>Y | No | SGRP | |
| s11-653750 | 345 | K>Q | No | SGRP | |
| s11-654117 | 467 | T>M | No | SGRP | |
| s11-654410 | 565 | L>F | No | SGRP | |
| s11-654495 | 593 | T>K | No | SGRP | |
| s11-654623 | 636 | S>P | No | SGRP | |
| s11-655113 | 799 | R>K | No | SGRP | |
| s11-655217 | 834 | C>R | No | SGRP | |
| s11-655266 | 850 | H>R | No | SGRP | |
| s11-655299 | 861 | W>* | No | SGRP | |
| s11-655304 | 863 | V>M | No | SGRP | |
| s11-655434 | 906 | R>K | No | SGRP | |
| s11-655479 | 921 | G>E | No | SGRP | |
| s11-655619 | 968 | S>A | No | SGRP | |
| s11-655678 | 987 | D>E | No | SGRP | |
| s11-655710 | 998 | L>R | No | SGRP | |
| s11-655748 | 1011 | L>I | No | SGRP | |
| s11-655763 | 1016 | I>V | No | SGRP | |
| s11-655772 | 1019 | L>F | No | SGRP | |
| s11-656252 | 1179 | R>G | No | SGRP | |
| s11-656374 | 1219 | S>Y | No | SGRP |
No associated diseases with P0CE68
5 regional properties for P0CE68
| Type | Name | Position | InterPro Accession |
|---|---|---|---|
| domain | ABC transporter-like, ATP-binding domain | 651 - 892 | IPR003439 |
| domain | AAA+ ATPase domain | 678 - 869 | IPR003593 |
| domain | ABC transporter type 1, transmembrane domain | 315 - 621 | IPR011527-1 |
| domain | ABC transporter type 1, transmembrane domain | 962 - 1218 | IPR011527-2 |
| conserved_site | ABC transporter-like, conserved site | 792 - 806 | IPR017871 |
3 GO annotations of cellular component
| Name | Definition |
|---|---|
| fungal-type vacuole membrane | The lipid bilayer surrounding a vacuole, the shape of which correlates with cell cycle phase. The membrane separates its contents from the cytoplasm of the cell. An example of this structure is found in Saccharomyces cerevisiae. |
| integral component of membrane | The component of a membrane consisting of the gene products and protein complexes having at least some part of their peptide sequence embedded in the hydrophobic region of the membrane. |
| membrane | A lipid bilayer along with all the proteins and protein complexes embedded in it an attached to it. |
3 GO annotations of molecular function
| Name | Definition |
|---|---|
| ABC-type transporter activity | Primary active transporter characterized by two nucleotide-binding domains and two transmembrane domains. Uses the energy generated from ATP hydrolysis to drive the transport of a substance across a membrane. |
| ATP binding | Binding to ATP, adenosine 5'-triphosphate, a universally important coenzyme and enzyme regulator. |
| ATPase-coupled transmembrane transporter activity | Primary active transporter of a solute across a membrane, via the reaction: ATP + H2O = ADP + phosphate, to directly drive the transport of a substance across a membrane. The transport protein may be transiently phosphorylated (P-type transporters), or not (ABC-type transporters and other families of transporters). Primary active transport occurs up the solute's concentration gradient and is driven by a primary energy source. |
2 GO annotations of biological process
| Name | Definition |
|---|---|
| macroautophagy | The major inducible pathway for the general turnover of cytoplasmic constituents in eukaryotic cells, it is also responsible for the degradation of active cytoplasmic enzymes and organelles during nutrient starvation. Macroautophagy involves the formation of double-membrane-bounded autophagosomes which enclose the cytoplasmic constituent targeted for degradation in a membrane-bounded structure. Autophagosomes then fuse with a lysosome (or vacuole) releasing single-membrane-bounded autophagic bodies that are then degraded within the lysosome (or vacuole). Some types of macroautophagy, e.g. pexophagy, mitophagy, involve selective targeting of the targets to be degraded. |
| transmembrane transport | The process in which a solute is transported across a lipid bilayer, from one side of a membrane to the other. |
9 homologous proteins in AiPD
| UniProt AC | Gene Name | Protein Name | Species | Evidence Code |
|---|---|---|---|---|
| P14772 | BPT1 | Bile pigment transporter 1 | Saccharomyces cerevisiae (strain ATCC 204508 / S288c) (Baker's yeast) | PR |
| P38735 | VMR1 | ABC transporter ATP-binding protein/permease VMR1 | Saccharomyces cerevisiae (strain ATCC 204508 / S288c) (Baker's yeast) | PR |
| Q96J66 | ABCC11 | ATP-binding cassette sub-family C member 11 | Homo sapiens (Human) | PR |
| Q80WJ6 | Abcc12 | ATP-binding cassette sub-family C member 12 | Mus musculus (Mouse) | PR |
| Q6Y306 | Abcc12 | ATP-binding cassette sub-family C member 12 | Rattus norvegicus (Rat) | PR |
| Q9C8H0 | ABCC12 | ABC transporter C family member 12 | Arabidopsis thaliana (Mouse-ear cress) | PR |
| Q9C8G9 | ABCC1 | ABC transporter C family member 1 | Arabidopsis thaliana (Mouse-ear cress) | PR |
| Q8VZZ4 | ABCC6 | ABC transporter C family member 6 | Arabidopsis thaliana (Mouse-ear cress) | PR |
| Q9M1C7 | ABCC9 | ABC transporter C family member 9 | Arabidopsis thaliana (Mouse-ear cress) | PR |
| 10 | 20 | 30 | 40 | 50 | 60 |
| MIKNGTCPYW | ERDDLSECAR | REYIEFKFPL | FILLTGMIYA | FCKVFRAFYL | RGKNHTNEAP |
| 70 | 80 | 90 | 100 | 110 | 120 |
| EFEEQGNGNH | EYARFSVLRL | KSAWESRSFC | NVNNRSTFDK | FKKFIEGAFI | VLQLTIHLYI |
| 130 | 140 | 150 | 160 | 170 | 180 |
| LSSMPMDNKK | FFHQGFLVQM | FLWILLLVVI | TLRLISASQS | FRWVLACKRD | LWAVSFYSYA |
| 190 | 200 | 210 | 220 | 230 | 240 |
| SLFTLSILPL | RSVFIGKIKD | KIMVKYIISE | TFIDLALLLL | LSTSSIEGTR | YSFLVENENK |
| 250 | 260 | 270 | 280 | 290 | 300 |
| KLPPAPTVFG | LLTFSRIDRL | IWKAYKHCLG | NADIWDLDIN | NKSIAILANF | EMSSKKGRLL |
| 310 | 320 | 330 | 340 | 350 | 360 |
| PNIICYFKAV | FISQLFLAFV | SSFLNFVPSL | LMPRILSYVN | DPKSKSWNLV | SLYVSSMLVS |
| 370 | 380 | 390 | 400 | 410 | 420 |
| KIIATTCRGQ | GLFLGEKGTM | QLRTVLISNI | YSKTLRRTIL | KDSTTSLQKN | ASTSFEENPD |
| 430 | 440 | 450 | 460 | 470 | 480 |
| SSEAEPRKKS | SRKDNSVNNV | MSIDAFKVSE | AMNTFYLACE | AVFMTVTALM | ILYSLLGWSA |
| 490 | 500 | 510 | 520 | 530 | 540 |
| FAGTFALLAM | IPLNFWCATF | YGNYQADQLI | LTDKRTSGIS | EALNSIRVIK | LLAWENLFYQ |
| 550 | 560 | 570 | 580 | 590 | 600 |
| KIINVRDGEI | RLLKKKATIF | FLNHLIWFFG | PTLVSAITFS | VFIKFQNQTL | TPTIAFTALS |
| 610 | 620 | 630 | 640 | 650 | 660 |
| LFAILRTPMD | QIASTVSLLI | QSFISLERIQ | DYLNESETRK | YEILEQSNTK | FGFEDASMEW |
| 670 | 680 | 690 | 700 | 710 | 720 |
| EAAETSFKLK | NISIDFKLNS | LNAIIGPTGS | GKSSLLLGLL | GELNLLSGKI | YVPTVESRDD |
| 730 | 740 | 750 | 760 | 770 | 780 |
| LEIGKDGMTN | SMAYCSQTPW | LISGTIKDNV | VFGEIFNKQK | FDDVMKSCCL | DKDIKAMTAG |
| 790 | 800 | 810 | 820 | 830 | 840 |
| IRTDVGDGGF | SLSGGQQQRI | ALARAIYSSS | RYLILDDCLS | AVDPETALYI | YEECLCGPMM |
| 850 | 860 | 870 | 880 | 890 | 900 |
| KGRTCIITSH | NISLVTKRAD | WLVILDRGEV | KSQGKPSDLI | KSNEFLRESI | NNDSKNTTHN |
| 910 | 920 | 930 | 940 | 950 | 960 |
| QIDLKRSTTS | KKTKNGDPEG | GNSQDEVCAE | VENFEETKME | GSVKFSAYKW | LADYFGGLGV |
| 970 | 980 | 990 | 1000 | 1010 | 1020 |
| VFVFTSSSIL | IHGITLSQGF | WLRYWLDTGS | SGSKSTWLYR | IVEGHSNIYF | LLTYIIIGLV |
| 1030 | 1040 | 1050 | 1060 | 1070 | 1080 |
| SSFLTSGKVW | IAIISGTNVT | KKIFAKLLSS | ILYAKLRFHN | VTPTGRIMNR | FSKDMDIIDQ |
| 1090 | 1100 | 1110 | 1120 | 1130 | 1140 |
| QLIPNFEGLS | YSVVVCLWII | LLIGYVTPQF | LLFAIPLCAL | YYTVCTLYLR | ASRELKRIDN |
| 1150 | 1160 | 1170 | 1180 | 1190 | 1200 |
| INISPIHQLF | AEAIKGVTTI | RALADERRFI | TQSLVAIDRS | NAPFFYLNMA | TEWITYRVDI |
| 1210 | |||||
| IGTLVLFSSS | VMIIMKAS |