P00782
Gene name |
apr |
Protein name |
Subtilisin BPN' |
Names |
Alkaline protease, Subtilisin DFE, Subtilisin Novo |
Species |
Bacillus amyloliquefaciens (Bacillus velezensis) |
KEGG Pathway |
|
EC number |
3.4.21.62: Serine endopeptidases |
Protein Class |
PEPTIDASE S8 (PTHR43806) |
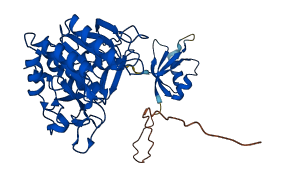
Descriptions
Serine protease subtilisin BPN (SBT) is secreted by the soil bacterium for the apparent biological function of digesting environmental proteins for bacterial absorption. The N-terminal prosegment domain forms a compact domain that binds SBT through an extensive interface involving the two parallel surface helices of enzyme, and binds the active site of SBT in a product-like manner, with Tyr77 in P1 binding pocket. This N-terminal 77 residues must be removed for the activation of this enzyme.
Autoinhibitory domains (AIDs)
Target domain |
132-360 (Subtilisin Carlsberg-like catalytic domain) |
Relief mechanism |
Cleavage |
Assay |
Mutagenesis experiment, Structural analysis |
Accessory elements
No accessory elements
Autoinhibited structure
Activated structure

64 structures for P00782
| Entry ID | Method | Resolution | Chain | Position | Source |
|---|---|---|---|---|---|
| 1A2Q | X-ray | 180 A | A | 108-382 | PDB |
| 1AK9 | X-ray | 180 A | A | 108-382 | PDB |
| 1AQN | X-ray | 180 A | A | 108-382 | PDB |
| 1AU9 | X-ray | 180 A | A | 108-382 | PDB |
| 1DUI | X-ray | 200 A | A | 108-382 | PDB |
| 1GNS | X-ray | 180 A | A | 112-382 | PDB |
| 1GNV | X-ray | 190 A | A | 108-382 | PDB |
| 1LW6 | X-ray | 150 A | E | 108-382 | PDB |
| 1S01 | X-ray | 170 A | A | 108-382 | PDB |
| 1S02 | X-ray | 190 A | A | 108-382 | PDB |
| 1SBH | X-ray | 180 A | A | 108-382 | PDB |
| 1SBI | X-ray | 220 A | A | 108-382 | PDB |
| 1SBN | X-ray | 210 A | E | 108-382 | PDB |
| 1SBT | X-ray | 250 A | A | 108-382 | PDB |
| 1SIB | X-ray | 240 A | E | 108-382 | PDB |
| 1SPB | X-ray | 200 A | PDB | ||
| 1ST2 | X-ray | 200 A | A | 108-382 | PDB |
| 1SUA | X-ray | 210 A | A | 108-382 | PDB |
| 1SUB | X-ray | 175 A | A | 108-382 | PDB |
| 1SUC | X-ray | 180 A | A | 108-382 | PDB |
| 1SUD | X-ray | 190 A | A | 108-382 | PDB |
| 1SUE | X-ray | 180 A | A | 108-382 | PDB |
| 1SUP | X-ray | 160 A | A | 108-382 | PDB |
| 1TM1 | X-ray | 170 A | E | 108-382 | PDB |
| 1TM3 | X-ray | 157 A | E | 108-382 | PDB |
| 1TM4 | X-ray | 170 A | E | 108-382 | PDB |
| 1TM5 | X-ray | 145 A | E | 108-382 | PDB |
| 1TM7 | X-ray | 159 A | E | 108-382 | PDB |
| 1TMG | X-ray | 167 A | E | 108-382 | PDB |
| 1TO1 | X-ray | 168 A | E | 108-382 | PDB |
| 1TO2 | X-ray | 130 A | E | 108-382 | PDB |
| 1UBN | X-ray | 240 A | A | 108-382 | PDB |
| 1V5I | X-ray | 150 A | A | 108-382 | PDB |
| 1Y1K | X-ray | 156 A | E | 108-382 | PDB |
| 1Y33 | X-ray | 180 A | E | 108-382 | PDB |
| 1Y34 | X-ray | 155 A | E | 108-382 | PDB |
| 1Y3B | X-ray | 180 A | E | 108-382 | PDB |
| 1Y3C | X-ray | 169 A | E | 108-382 | PDB |
| 1Y3D | X-ray | 180 A | E | 108-382 | PDB |
| 1Y3F | X-ray | 172 A | E | 108-382 | PDB |
| 1Y48 | X-ray | 184 A | E | 108-382 | PDB |
| 1Y4A | X-ray | 160 A | E | 108-382 | PDB |
| 1Y4D | X-ray | 200 A | E | 108-382 | PDB |
| 1YJA | X-ray | 180 A | A | 108-382 | PDB |
| 1YJB | X-ray | 180 A | A | 108-382 | PDB |
| 1YJC | X-ray | 180 A | A | 108-382 | PDB |
| 2SBT | X-ray | 280 A | A | 108-382 | PDB |
| 2SIC | X-ray | 180 A | E | 108-382 | PDB |
| 2SNI | X-ray | 210 A | E | 108-382 | PDB |
| 2ST1 | X-ray | 180 A | A | 108-382 | PDB |
| 3BGO | X-ray | 180 A | PDB | ||
| 3CNQ | X-ray | 171 A | PDB | ||
| 3CO0 | X-ray | 193 A | PDB | ||
| 3F49 | X-ray | 170 A | S | 108-382 | PDB |
| 3SIC | X-ray | 180 A | E | 108-382 | PDB |
| 5OX2 | X-ray | 224 A | A | 108-382 | PDB |
| 5SIC | X-ray | 220 A | E | 108-382 | PDB |
| 7AM3 | X-ray | 161 A | A | 108-382 | PDB |
| 7AM4 | X-ray | 181 A | A | 108-382 | PDB |
| 7AM5 | X-ray | 230 A | A | 108-382 | PDB |
| 7AM6 | X-ray | 270 A | A/B/C | 108-382 | PDB |
| 7AM7 | X-ray | 261 A | A/B/C | 108-382 | PDB |
| 7AM8 | X-ray | 204 A | A | 108-382 | PDB |
| AF-P00782-F1 | Predicted | AlphaFoldDB |
1 variants for P00782
| Variant ID(s) | Position | Change | Description | Diseaes Association | Provenance |
|---|---|---|---|---|---|
| 128 | Y>F | strain: DC-4 [UniProt] | No |
No associated diseases with P00782
6 regional properties for P00782
| Type | Name | Position | InterPro Accession |
|---|---|---|---|
| domain | Peptidase S8/S53 domain | 130 - 366 | IPR000209 |
| domain | Peptidase S8 propeptide/proteinase inhibitor I9 | 39 - 105 | IPR010259 |
| active_site | Peptidase S8, subtilisin, His-active site | 171 - 181 | IPR022398 |
| active_site | Peptidase S8, subtilisin, Asp-active site | 135 - 146 | IPR023827 |
| active_site | Peptidase S8, subtilisin, Ser-active site | 326 - 336 | IPR023828 |
| domain | Subtilisin Carlsberg-like catalytic domain | 132 - 360 | IPR034202 |
Functions
| Description | ||
|---|---|---|
| EC Number | 3.4.21.62 | Serine endopeptidases |
| Subcellular Localization |
|
|
| PANTHER Family | PTHR43806 | PEPTIDASE S8 |
| PANTHER Subfamily | PTHR43806:SF59 | CEREVISIN-RELATED |
| PANTHER Protein Class |
serine protease
protease |
|
| PANTHER Pathway Category | No pathway information available | |
1 GO annotations of cellular component
| Name | Definition |
|---|---|
| extracellular region | The space external to the outermost structure of a cell. For cells without external protective or external encapsulating structures this refers to space outside of the plasma membrane. This term covers the host cell environment outside an intracellular parasite. |
2 GO annotations of molecular function
| Name | Definition |
|---|---|
| metal ion binding | Binding to a metal ion. |
| serine-type endopeptidase activity | Catalysis of the hydrolysis of internal, alpha-peptide bonds in a polypeptide chain by a catalytic mechanism that involves a catalytic triad consisting of a serine nucleophile that is activated by a proton relay involving an acidic residue (e.g. aspartate or glutamate) and a basic residue (usually histidine). |
3 GO annotations of biological process
| Name | Definition |
|---|---|
| fibrinolysis | A process that solubilizes fibrin in the bloodstream of a multicellular organism, chiefly by the proteolytic action of plasmin. |
| proteolysis | The hydrolysis of proteins into smaller polypeptides and/or amino acids by cleavage of their peptide bonds. |
| sporulation resulting in formation of a cellular spore | The process in which a relatively unspecialized cell acquires the specialized features of a cellular spore, a cell form that can be used for dissemination, for survival of adverse conditions because of its heat and dessication resistance, and/or for reproduction. |
No homologous proteins in AiPD
| UniProt AC | Gene Name | Protein Name | Species | Evidence Code |
|---|---|---|---|---|
| No homologous proteins | ||||
| 10 | 20 | 30 | 40 | 50 | 60 |
| MRGKKVWISL | LFALALIFTM | AFGSTSSAQA | AGKSNGEKKY | IVGFKQTMST | MSAAKKKDVI |
| 70 | 80 | 90 | 100 | 110 | 120 |
| SEKGGKVQKQ | FKYVDAASAT | LNEKAVKELK | KDPSVAYVEE | DHVAHAYAQS | VPYGVSQIKA |
| 130 | 140 | 150 | 160 | 170 | 180 |
| PALHSQGYTG | SNVKVAVIDS | GIDSSHPDLK | VAGGASMVPS | ETNPFQDNNS | HGTHVAGTVA |
| 190 | 200 | 210 | 220 | 230 | 240 |
| ALNNSIGVLG | VAPSASLYAV | KVLGADGSGQ | YSWIINGIEW | AIANNMDVIN | MSLGGPSGSA |
| 250 | 260 | 270 | 280 | 290 | 300 |
| ALKAAVDKAV | ASGVVVVAAA | GNEGTSGSSS | TVGYPGKYPS | VIAVGAVDSS | NQRASFSSVG |
| 310 | 320 | 330 | 340 | 350 | 360 |
| PELDVMAPGV | SIQSTLPGNK | YGAYNGTSMA | SPHVAGAAAL | ILSKHPNWTN | TQVRSSLENT |
| 370 | 380 | ||||
| TTKLGDSFYY | GKGLINVQAA | AQ |