O89040
Gene name |
Plcb2 |
Protein name |
1-phosphatidylinositol 4,5-bisphosphate phosphodiesterase beta-2 |
Names |
Phosphoinositide phospholipase C-beta-2, Phospholipase C-beta-2, PLC-beta-2 |
Species |
Rattus norvegicus (Rat) |
KEGG Pathway |
rno:85240 |
EC number |
3.1.4.11: Phosphoric diester hydrolases |
Protein Class |
|
Descriptions
(Annotation based on sequence homology with Q00722)
PLCB2 encodes for 1-phosphatidylinositol 4,5-bisphosphate phosphodiesterase beta-2, hydrolyzes phosphatidylinositol 4,5-bisphosphate (PtdIns[4,5]P2) into the second messengers diacylglycerol (DAG) and inositol 1,4,5-trisphosphate (IP3). Autoinhibitory region is a portion of the linker that separates the conserved X and Y boxes comprising the catalytic TIM barrel, at residues 466-537, and occludes the active site of PLCB2. Specifically, the ordered region of X/Y linker at residues 516-535 seems to fine-tune access to the active site, in which deletion of this region only gave partial activation of PLCB2. However, the disordered region of X/Y linker at residues 470-515 is the major autoinhibitory region, in which deletion of this region enhanced activity by 10-fold higher basal activity relative to the ordered region.
Autoinhibitory domains (AIDs)
Target domain |
|
Relief mechanism |
|
Assay |
cis-regPred |
Accessory elements
No accessory elements
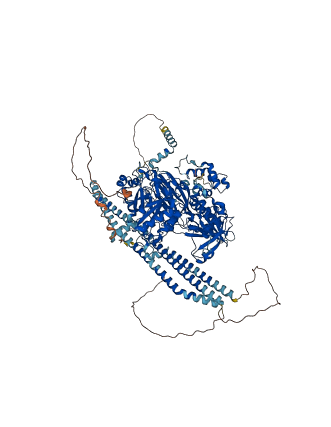
Autoinhibited structure

Activated structure

1 structures for O89040
| Entry ID | Method | Resolution | Chain | Position | Source |
|---|---|---|---|---|---|
| AF-O89040-F1 | Predicted | AlphaFoldDB |
7 variants for O89040
| Variant ID(s) | Position | Change | Description | Diseaes Association | Provenance |
|---|---|---|---|---|---|
| rs3319985897 | 1015 | M>I | No | EVA | |
| rs105561320 | 1020 | R>I | No | EVA | |
| rs105647278 | 1030 | F>L | No | EVA | |
| rs106050987 | 1033 | T>P | No | EVA | |
| rs105854020 | 1036 | T>P | No | EVA | |
| rs3320002478 | 1140 | M>I | No | EVA | |
| rs3319889292 | 1146 | A>V | No | EVA |
No associated diseases with O89040
8 regional properties for O89040
| Type | Name | Position | InterPro Accession |
|---|---|---|---|
| domain | C2 domain | 666 - 794 | IPR000008 |
| domain | Phosphatidylinositol-specific phospholipase C, X domain | 312 - 464 | IPR000909 |
| domain | Phospholipase C, phosphatidylinositol-specific, Y domain | 550 - 666 | IPR001711 |
| domain | Phospholipase C-beta, C-terminal domain | 977 - 1151 | IPR014815 |
| domain | Phosphoinositide-specific phospholipase C, EF-hand-like domain | 214 - 303 | IPR015359 |
| domain | 1-phosphatidylinositol 4,5-bisphosphate phosphodiesterase beta-2, catalytic domain | 311 - 653 | IPR028403 |
| domain | PLC-beta, PH domain | 12 - 144 | IPR037862 |
| domain | 1-phosphatidylinositol 4,5-bisphosphate phosphodiesterase beta-2, EF-hand domain | 149 - 299 | IPR046969 |
Functions
| Description | ||
|---|---|---|
| EC Number | 3.1.4.11 | Phosphoric diester hydrolases |
| Subcellular Localization |
|
|
| PANTHER Family | ||
| PANTHER Subfamily | ||
| PANTHER Protein Class | ||
| PANTHER Pathway Category | No pathway information available | |
5 GO annotations of cellular component
| Name | Definition |
|---|---|
| cytoplasm | The contents of a cell excluding the plasma membrane and nucleus, but including other subcellular structures. |
| cytosol | The part of the cytoplasm that does not contain organelles but which does contain other particulate matter, such as protein complexes. |
| G-protein beta/gamma-subunit complex | The heterodimer formed by the beta and gamma subunits of a heterotrimeric G protein, which dissociates from the alpha subunit upon guanine nuclotide exchange. |
| Golgi apparatus | A membrane-bound cytoplasmic organelle of the endomembrane system that further processes the core oligosaccharides (e.g. N-glycans) added to proteins in the endoplasmic reticulum and packages them into membrane-bound vesicles. The Golgi apparatus operates at the intersection of the secretory, lysosomal, and endocytic pathways. |
| plasma membrane | The membrane surrounding a cell that separates the cell from its external environment. It consists of a phospholipid bilayer and associated proteins. |
5 GO annotations of molecular function
| Name | Definition |
|---|---|
| calcium ion binding | Binding to a calcium ion (Ca2+). |
| G-protein beta/gamma-subunit complex binding | Binding to a complex of G-protein beta/gamma subunits. |
| phosphatidylinositol phospholipase C activity | Catalysis of the reaction: 1-phosphatidyl-1D-myo-inositol 4,5-bisphosphate + H(2)O = 1,2-diacylglycerol + 1D-myo-inositol 1,4,5-trisphosphate + H(+). |
| phospholipase C activity | Catalysis of the reaction: a phospholipid + H2O = 1,2-diacylglycerol + a phosphatidate. |
| phospholipid binding | Binding to a phospholipid, a class of lipids containing phosphoric acid as a mono- or diester. |
6 GO annotations of biological process
| Name | Definition |
|---|---|
| detection of chemical stimulus involved in sensory perception of bitter taste | The series of events required for a bitter taste stimulus to be received and converted to a molecular signal. |
| lipid catabolic process | The chemical reactions and pathways resulting in the breakdown of lipids, compounds soluble in an organic solvent but not, or sparingly, in an aqueous solvent. |
| phosphatidylinositol metabolic process | The chemical reactions and pathways involving phosphatidylinositol, any glycophospholipid in which a sn-glycerol 3-phosphate residue is esterified to the 1-hydroxyl group of 1D-myo-inositol. |
| phosphatidylinositol-mediated signaling | The series of molecular signals in which a cell uses a phosphatidylinositol-mediated signaling to convert a signal into a response. Phosphatidylinositols include phosphatidylinositol (PtdIns) and its phosphorylated derivatives. |
| phospholipase C-activating G protein-coupled receptor signaling pathway | A G protein-coupled receptor signaling pathway in which the signal is transmitted via the activation of phospholipase C (PLC) and a subsequent increase in the intracellular concentration of inositol trisphosphate (IP3) and diacylglycerol (DAG). |
| sensory perception of bitter taste | The series of events required to receive a bitter taste stimulus, convert it to a molecular signal, and recognize and characterize the signal. This is a neurological process. |
16 homologous proteins in AiPD
| UniProt AC | Gene Name | Protein Name | Species | Evidence Code |
|---|---|---|---|---|
| P32383 | PLC1 | 1-phosphatidylinositol 4,5-bisphosphate phosphodiesterase 1 | Saccharomyces cerevisiae (strain ATCC 204508 / S288c) (Baker's yeast) | PR |
| Q00722 | PLCB2 | 1-phosphatidylinositol 4,5-bisphosphate phosphodiesterase beta-2 | Homo sapiens (Human) | EV |
| A3KGF7 | Plcb2 | 1-phosphatidylinositol 4,5-bisphosphate phosphodiesterase beta-2 | Mus musculus (Mouse) | PR |
| P24135 | Plcg2 | 1-phosphatidylinositol 4,5-bisphosphate phosphodiesterase gamma-2 | Rattus norvegicus (Rat) | SS |
| P10686 | Plcg1 | 1-phosphatidylinositol 4,5-bisphosphate phosphodiesterase gamma-1 | Rattus norvegicus (Rat) | EV |
| P10687 | Plcb1 | 1-phosphatidylinositol 4,5-bisphosphate phosphodiesterase beta-1 | Rattus norvegicus (Rat) | SS |
| Q99P84 | Plce1 | 1-phosphatidylinositol 4,5-bisphosphate phosphodiesterase epsilon-1 | Rattus norvegicus (Rat) | EV |
| P10688 | Plcd1 | 1-phosphatidylinositol 4,5-bisphosphate phosphodiesterase delta-1 | Rattus norvegicus (Rat) | SS |
| Q99JE6 | Plcb3 | 1-phosphatidylinositol 4,5-bisphosphate phosphodiesterase beta-3 | Rattus norvegicus (Rat) | SS |
| Q39032 | PLC1 | Phosphoinositide phospholipase C 1 | Arabidopsis thaliana (Mouse-ear cress) | PR |
| Q944C2 | PLC5 | Phosphoinositide phospholipase C 5 | Arabidopsis thaliana (Mouse-ear cress) | PR |
| Q9STZ3 | PLC8 | Phosphoinositide phospholipase C 8 | Arabidopsis thaliana (Mouse-ear cress) | PR |
| Q8GV43 | PLC6 | Phosphoinositide phospholipase C 6 | Arabidopsis thaliana (Mouse-ear cress) | PR |
| Q56W08 | PLC3 | Phosphoinositide phospholipase C 3 | Arabidopsis thaliana (Mouse-ear cress) | PR |
| Q6NMA7 | PLC9 | Phosphoinositide phospholipase C 9 | Arabidopsis thaliana (Mouse-ear cress) | PR |
| Q944C1 | PLC4 | Phosphoinositide phospholipase C 4 | Arabidopsis thaliana (Mouse-ear cress) | PR |
| 10 | 20 | 30 | 40 | 50 | 60 |
| MSLLNPVLLP | PKVKAYLSQG | ERFIKWDDET | SIASPVILRV | DPKGYYLYWT | HQSKEMEFLD |
| 70 | 80 | 90 | 100 | 110 | 120 |
| VTSIRDTRFG | KFAKIPKSQK | LREVFNMDFP | DNHFLLKTFT | VVSGPDMVGL | TFHNFVSYKE |
| 130 | 140 | 150 | 160 | 170 | 180 |
| NVGKDWAEDV | LALAKHPMTA | NASRSTFLDK | ILVKLKMQLS | PEGKIPVKNF | FQMFPADRKR |
| 190 | 200 | 210 | 220 | 230 | 240 |
| VEAALSACHL | AKGKNDAINP | EDFPESVYKS | FLMSLCPRPE | IDEIFTSYHA | KAKPYMTKEH |
| 250 | 260 | 270 | 280 | 290 | 300 |
| LTKFINQKQR | DPRLNSLLFP | PARPEQVQAL | IDKYEPSGIN | VQRGQLSPEG | MVWFLCGPEN |
| 310 | 320 | 330 | 340 | 350 | 360 |
| SVLAHDTLRI | HQDMTQPLNH | YFINSSHNTY | LTAGQFSGPS | SAEMYRQVLL | SGCRCVELDC |
| 370 | 380 | 390 | 400 | 410 | 420 |
| WKGKPPDEEP | IITHGFTMTT | DILFKEAVEA | IAESAFKTSP | YPVILSFENH | VDSPRQQAKM |
| 430 | 440 | 450 | 460 | 470 | 480 |
| AEYCRTMFGE | TLLTEPLENF | PLKPGMPLPS | PEDLRGKILI | KNKKNQFSGP | ASPNKKPDGV |
| 490 | 500 | 510 | 520 | 530 | 540 |
| SEGGFPSSVP | VEEDTGWTAE | DRTEVEEGEE | EEEVEEEEEE | ESGNLDEEEI | KKMQSDEGTA |
| 550 | 560 | 570 | 580 | 590 | 600 |
| GLEVTAYEEM | SSLVNYIQPT | KFISFEFSAQ | KNRSYLVSSF | TELKAYELLS | KASMQFVDYN |
| 610 | 620 | 630 | 640 | 650 | 660 |
| KRQMSRVYPK | GTRMDSSNYM | PQMFWNAGCQ | MVALNFQTMD | LPMQQNMALF | EFNGQSGYLL |
| 670 | 680 | 690 | 700 | 710 | 720 |
| KHEFMRRQDK | QFNPFSVDRI | DVVVATTLSI | TVISGQFLSE | RSVRTYVEVE | LFGLPGDPKR |
| 730 | 740 | 750 | 760 | 770 | 780 |
| RYRTKLSPTA | NSINPVWKEE | PFIFEKILVP | ELASLRIAVM | EEGGKFIGHR | IIPINALHSG |
| 790 | 800 | 810 | 820 | 830 | 840 |
| YHHLCLRSES | NMPLTMPALF | VFLEMKDYVP | DTWADLTVAL | ANPIKYFSAH | DKKSVKLKEV |
| 850 | 860 | 870 | 880 | 890 | 900 |
| TGSLPEKLFS | GIPVASQSNG | APVSAGNGST | APGTKAKEEA | TKEVAEPQTT | SLEELRELKG |
| 910 | 920 | 930 | 940 | 950 | 960 |
| VVKLQRRHEK | ELRELERRGA | RRWEELLQRG | AAQLAELQDP | AASCKLRPGK | GSRKKRIVPC |
| 970 | 980 | 990 | 1000 | 1010 | 1020 |
| EETIVVPREV | LEGPDPRVQD | LKDRLEQELQ | QQGEEQYRSV | LKRKEQHVTE | QIAKMMELAR |
| 1030 | 1040 | 1050 | 1060 | 1070 | 1080 |
| EKQAAELKSF | KETSETDTKE | MKKKLEAKRL | ERIQAMTKVT | TDKVAQERLK | REINNSHIQE |
| 1090 | 1100 | 1110 | 1120 | 1130 | 1140 |
| VVQAVKQMTE | TLERHQEKLE | EKQTACLEQI | QAMEKQFQEK | ALAEYEAKMK | GLEAEVKESM |
| 1150 | 1160 | 1170 | 1180 | ||
| RACFKACFPT | EAEEKPERPC | EASEESCPQE | PLVNKTDTQE | SRL |