O88704
Gene name |
Hcn1 (Bcng1, Hac2) |
Protein name |
Potassium/sodium hyperpolarization-activated cyclic nucleotide-gated channel 1 |
Names |
Brain cyclic nucleotide-gated channel 1 , BCNG-1 , Hyperpolarization-activated cation channel 2 , HAC-2 |
Species |
Mus musculus (Mouse) |
KEGG Pathway |
mmu:15165 |
EC number |
|
Protein Class |
|
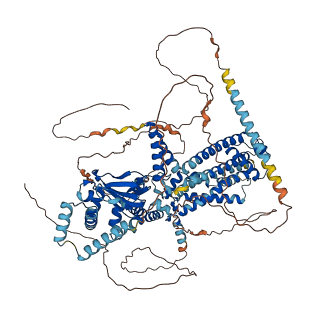
Descriptions
Autoinhibitory domains (AIDs)
Target domain |
132-391 (Ion transport domain) |
Relief mechanism |
Ligand binding |
Assay |
|
Target domain |
132-391 (Ion transport domain) |
Relief mechanism |
Ligand binding |
Assay |
|
Accessory elements
No accessory elements
References
- Yeon JH et al. (2016) "Systems-wide Identification of cis-Regulatory Elements in Proteins", Cell systems, 2, 89-100
- Wicks NL et al. (2011) "Cytoplasmic cAMP-sensing domain of hyperpolarization-activated cation (HCN) channels uses two structurally distinct mechanisms to regulate voltage gating", Proceedings of the National Academy of Sciences of the United States of America, 108, 609-14
- Akimoto M et al. (2014) "A mechanism for the auto-inhibition of hyperpolarization-activated cyclic nucleotide-gated (HCN) channel opening and its relief by cAMP", The Journal of biological chemistry, 289, 22205-20
- Xu X et al. (2010) "Structural basis for the cAMP-dependent gating in the human HCN4 channel", The Journal of biological chemistry, 285, 37082-91
Autoinhibited structure

Activated structure

2 structures for O88704
| Entry ID | Method | Resolution | Chain | Position | Source |
|---|---|---|---|---|---|
| 3U0Z | X-ray | 290 A | A/B | 390-592 | PDB |
| AF-O88704-F1 | Predicted | AlphaFoldDB |
28 variants for O88704
| Variant ID(s) | Position | Change | Description | Diseaes Association | Provenance |
|---|---|---|---|---|---|
| rs3389277730 | 250 | K>T | No | EVA | |
| rs3389306683 | 256 | R>L | No | EVA | |
| rs3389287658 | 256 | R>W | No | EVA | |
| rs3389304327 | 260 | L>R | No | EVA | |
| rs3404480023 | 291 | I>S | No | EVA | |
| rs1132792937 | 332 | K>N | No | EVA | |
| rs1135268988 | 333 | Q>H | No | EVA | |
| rs1132593574 | 334 | Y>N | No | EVA | |
| rs1132997530 | 341 | A>G | No | EVA | |
| rs1134951338 | 342 | M>V | No | EVA | |
| rs1131865219 | 343 | S>G | No | EVA | |
| rs1134287979 | 345 | M>K | No | EVA | |
| rs3389232420 | 400 | Y>H | No | EVA | |
| rs3389262381 | 519 | M>T | No | EVA | |
| rs3389277737 | 535 | K>N | No | EVA | |
| rs3389306463 | 551 | Y>F | No | EVA | |
| rs3389270922 | 556 | D>N | No | EVA | |
| rs3389262417 | 564 | E>D | No | EVA | |
| rs3411154752 | 587 | N>K | No | EVA | |
| rs3389277692 | 606 | Q>H | No | EVA | |
| rs3389301974 | 623 | Q>E | No | EVA | |
| rs3389302019 | 628 | I>N | No | EVA | |
| rs3389270988 | 631 | P>R | No | EVA | |
| rs241512417 | 645 | P>L | No | EVA | |
| rs3389262353 | 673 | S>N | No | EVA | |
| rs3389271004 | 702 | A>D | No | EVA | |
| rs265986383 | 722 | P>T | No | EVA | |
| rs4230116 | 727 | P>Q | No | EVA |
No associated diseases with O88704
4 regional properties for O88704
15 GO annotations of cellular component
| Name | Definition |
|---|---|
| apical dendrite | A dendrite that emerges near the apical pole of a neuron. In bipolar neurons, apical dendrites are located on the opposite side of the soma from the axon. |
| axon | The long process of a neuron that conducts nerve impulses, usually away from the cell body to the terminals and varicosities, which are sites of storage and release of neurotransmitter. |
| axon terminus | Terminal inflated portion of the axon, containing the specialized apparatus necessary to release neurotransmitters. The axon terminus is considered to be the whole region of thickening and the terminal button is a specialized region of it. |
| basolateral plasma membrane | The region of the plasma membrane that includes the basal end and sides of the cell. Often used in reference to animal polarized epithelial membranes, where the basal membrane is the part attached to the extracellular matrix, or in plant cells, where the basal membrane is defined with respect to the zygotic axis. |
| cell surface | The external part of the cell wall and/or plasma membrane. |
| dendrite | A neuron projection that has a short, tapering, morphology. Dendrites receive and integrate signals from other neurons or from sensory stimuli, and conduct nerve impulses towards the axon or the cell body. In most neurons, the impulse is conveyed from dendrites to axon via the cell body, but in some types of unipolar neuron, the impulse does not travel via the cell body. |
| dendrite membrane | The portion of the plasma membrane surrounding a dendrite. |
| dendritic shaft | Cylindric portion of the dendrite, directly stemming from the perikaryon, and carrying the dendritic spines. |
| glutamatergic synapse | A synapse that uses glutamate as a neurotransmitter. |
| HCN channel complex | A cation ion channel with a preference for K+ over Na+ ions, which is activated by membrane hyperpolarization, and consists of a tetramer of HCN family members. Some members of this family (HCN1, HCN2 and HCN4) are also activated when cAMP binds to their cyclic nucleotide binding domain (CNBD). Channel complexes of this family play an important role in the control of pacemaker activity in the heart. |
| neuronal cell body | The portion of a neuron that includes the nucleus, but excludes cell projections such as axons and dendrites. |
| plasma membrane | The membrane surrounding a cell that separates the cell from its external environment. It consists of a phospholipid bilayer and associated proteins. |
| postsynaptic membrane | A specialized area of membrane facing the presynaptic membrane on the tip of the nerve ending and separated from it by a minute cleft (the synaptic cleft). Neurotransmitters cross the synaptic cleft and transmit the signal to the postsynaptic membrane. |
| presynaptic active zone membrane | The membrane portion of the presynaptic active zone; it is the site where docking and fusion of synaptic vesicles occurs for the release of neurotransmitters. |
| somatodendritic compartment | The region of a neuron that includes the cell body (cell soma) and dendrite(s), but excludes the axon. |
9 GO annotations of molecular function
| Name | Definition |
|---|---|
| cAMP binding | Binding to cAMP, the nucleotide cyclic AMP (adenosine 3',5'-cyclophosphate). |
| identical protein binding | Binding to an identical protein or proteins. |
| intracellular cAMP-activated cation channel activity involved in regulation of presynaptic membrane potential | Enables the transmembrane transfer of a cation by a channel that opens when intracellular cAMP has been bound by the channel complex or one of its constituent parts, to regulate the presynaptic membrane potential. |
| intracellularly cAMP-activated cation channel activity | Enables the transmembrane transfer of a cation by a channel that opens when intracellular cAMP has been bound by the channel complex or one of its constituent parts. |
| phosphatidylinositol-3,4,5-trisphosphate binding | Binding to phosphatidylinositol-3,4,5-trisphosphate, a derivative of phosphatidylinositol in which the inositol ring is phosphorylated at the 3', 4' and 5' positions. |
| phosphatidylinositol-4,5-bisphosphate binding | Binding to phosphatidylinositol-4,5-bisphosphate, a derivative of phosphatidylinositol in which the inositol ring is phosphorylated at the 4' and 5' positions. |
| voltage-gated monoatomic cation channel activity | Enables the transmembrane transfer of a cation by a voltage-gated channel. A cation is a positively charged ion. A voltage-gated channel is a channel whose open state is dependent on the voltage across the membrane in which it is embedded. |
| voltage-gated potassium channel activity | Enables the transmembrane transfer of a potassium ion by a voltage-gated channel. A voltage-gated channel is a channel whose open state is dependent on the voltage across the membrane in which it is embedded. |
| voltage-gated sodium channel activity | Enables the transmembrane transfer of a sodium ion by a voltage-gated channel. A voltage-gated channel is a channel whose open state is dependent on the voltage across the membrane in which it is embedded. |
14 GO annotations of biological process
| Name | Definition |
|---|---|
| apical protein localization | Any process in which a protein is transported to, or maintained in, apical regions of the cell. |
| cellular response to cAMP | Any process that results in a change in state or activity of a cell (in terms of movement, secretion, enzyme production, gene expression, etc.) as a result of a cAMP (cyclic AMP, adenosine 3',5'-cyclophosphate) stimulus. |
| general adaptation syndrome, behavioral process | The set of behavioral processes that occur as part of the general adaptation syndrome, the response of the body to a strong, stressful stimulus. |
| negative regulation of action potential | Any process that stops, prevents, or reduces the frequency, rate or extent of action potential creation, propagation or termination. This typically occurs via modulation of the activity or expression of voltage-gated ion channels. |
| neuronal action potential | An action potential that occurs in a neuron. |
| positive regulation of membrane hyperpolarization | Any process that activates or increases the frequency, rate or extent of membrane hyperpolarization. |
| potassium ion transmembrane transport | A process in which a potassium ion is transported from one side of a membrane to the other. |
| protein homotetramerization | The formation of a protein homotetramer, a macromolecular structure consisting of four noncovalently associated identical subunits. |
| regulation of membrane depolarization | Any process that modulates the rate, frequency or extent of membrane depolarization. Membrane depolarization is the process in which membrane potential changes in the depolarizing direction from the resting potential, usually from negative to positive. |
| regulation of membrane hyperpolarization | Any process that modulates the frequency, rate or extent of membrane hyperpolarization. |
| regulation of postsynaptic membrane potential | Any process that modulates the potential difference across a post-synaptic membrane. |
| response to calcium ion | Any process that results in a change in state or activity of a cell or an organism (in terms of movement, secretion, enzyme production, gene expression, etc.) as a result of a calcium ion stimulus. |
| retinal cone cell development | Development of a cone cell, one of the sensory cells in the eye that reacts to the presence of light. Cone cells contain the photopigment iodopsin or cyanopsin and are responsible for photopic (daylight) vision. |
| sodium ion transmembrane transport | A process in which a sodium ion is transported from one side of a membrane to the other by means of some agent such as a transporter or pore. |
18 homologous proteins in AiPD
| UniProt AC | Gene Name | Protein Name | Species | Evidence Code |
|---|---|---|---|---|
| Q9UL51 | HCN2 | Potassium/sodium hyperpolarization-activated cyclic nucleotide-gated channel 2 | Homo sapiens (Human) | SS |
| Q9Y3Q4 | HCN4 | Potassium/sodium hyperpolarization-activated cyclic nucleotide-gated channel 4 | Homo sapiens (Human) | EV |
| Q9P1Z3 | HCN3 | Potassium/sodium hyperpolarization-activated cyclic nucleotide-gated channel 3 | Homo sapiens (Human) | SS |
| O60741 | HCN1 | Potassium/sodium hyperpolarization-activated cyclic nucleotide-gated channel 1 | Homo sapiens (Human) | SS |
| O88703 | Hcn2 | Potassium/sodium hyperpolarization-activated cyclic nucleotide-gated channel 2 | Mus musculus (Mouse) | EV |
| O88705 | Hcn3 | Potassium/sodium hyperpolarization-activated cyclic nucleotide-gated channel 3 | Mus musculus (Mouse) | SS |
| O70507 | Hcn4 | Potassium/sodium hyperpolarization-activated cyclic nucleotide-gated channel 4 | Mus musculus (Mouse) | SS |
| Q9JKA9 | Hcn2 | Potassium/sodium hyperpolarization-activated cyclic nucleotide-gated channel 2 | Rattus norvegicus (Rat) | SS |
| Q9JKA7 | Hcn4 | Potassium/sodium hyperpolarization-activated cyclic nucleotide-gated channel 4 | Rattus norvegicus (Rat) | SS |
| Q9JKA8 | Hcn3 | Potassium/sodium hyperpolarization-activated cyclic nucleotide-gated channel 3 | Rattus norvegicus (Rat) | SS |
| Q9JKB0 | Hcn1 | Potassium/sodium hyperpolarization-activated cyclic nucleotide-gated channel 1 | Rattus norvegicus (Rat) | SS |
| A2ZX97 | Os01g0718700 | Potassium channel KAT6 | Oryza sativa subsp japonica (Rice) | PR |
| Q6K3T2 | Os02g0245800 | Potassium channel KAT1 | Oryza sativa subsp japonica (Rice) | PR |
| Q9SKD7 | CNGC3 | Probable cyclic nucleotide-gated ion channel 3 | Arabidopsis thaliana (Mouse-ear cress) | PR |
| Q9LDR2 | CNGC19 | Putative cyclic nucleotide-gated ion channel 19 | Arabidopsis thaliana (Mouse-ear cress) | PR |
| Q9SU64 | CNGC16 | Probable cyclic nucleotide-gated ion channel 16 | Arabidopsis thaliana (Mouse-ear cress) | PR |
| P92960 | ATHB-4 | Potassium channel KAT3 | Arabidopsis thaliana (Mouse-ear cress) | PR |
| Q39128 | KAT1 | Potassium channel KAT1 | Arabidopsis thaliana (Mouse-ear cress) | PR |
| 10 | 20 | 30 | 40 | 50 | 60 |
| MEGGGKPNSA | SNSRDDGNSV | FPSKAPATGP | VAADKRLGTP | PGGGAAGKEH | GNSVCFKVDG |
| 70 | 80 | 90 | 100 | 110 | 120 |
| GGGEEPAGSF | EDAEGPRRQY | GFMQRQFTSM | LQPGVNKFSL | RMFGSQKAVE | KEQERVKTAG |
| 130 | 140 | 150 | 160 | 170 | 180 |
| FWIIHPYSDF | RFYWDLIMLI | MMVGNLVIIP | VGITFFTEQT | TTPWIIFNVA | SDTVFLLDLI |
| 190 | 200 | 210 | 220 | 230 | 240 |
| MNFRTGTVNE | DSSEIILDPK | VIKMNYLKSW | FVVDFISSIP | VDYIFLIVEK | GMDSEVYKTA |
| 250 | 260 | 270 | 280 | 290 | 300 |
| RALRIVRFTK | ILSLLRLLRL | SRLIRYIHQW | EEIFHMTYDL | ASAVVRIFNL | IGMMLLLCHW |
| 310 | 320 | 330 | 340 | 350 | 360 |
| DGCLQFLVPL | LQDFPPDCWV | SLNEMVNDSW | GKQYSYALFK | AMSHMLCIGY | GAQAPVSMSD |
| 370 | 380 | 390 | 400 | 410 | 420 |
| LWITMLSMIV | GATCYAMFVG | HATALIQSLD | SSRRQYQEKY | KQVEQYMSFH | KLPADMRQKI |
| 430 | 440 | 450 | 460 | 470 | 480 |
| HDYYEHRYQG | KIFDEENILS | ELNDPLREEI | VNFNCRKLVA | TMPLFANADP | NFVTAMLSKL |
| 490 | 500 | 510 | 520 | 530 | 540 |
| RFEVFQPGDY | IIREGAVGKK | MYFIQHGVAG | VITKSSKEMK | LTDGSYFGEI | CLLTKGRRTA |
| 550 | 560 | 570 | 580 | 590 | 600 |
| SVRADTYCRL | YSLSVDNFNE | VLEEYPMMRR | AFETVAIDRL | DRIGKKNSIL | LQKFQKDLNT |
| 610 | 620 | 630 | 640 | 650 | 660 |
| GVFNNQENEI | LKQIVKHDRE | MVQAIPPINY | PQMTALNCTS | STTTPTSRMR | TQSPPVYTAT |
| 670 | 680 | 690 | 700 | 710 | 720 |
| SLSHSNLHSP | SPSTQTPQPS | AILSPCSYTT | AVCSPPIQSP | LATRTFHYAS | PTASQLSLMQ |
| 730 | 740 | 750 | 760 | 770 | 780 |
| QPQQQLPQSQ | VQQTQTQTQQ | QQQQQQQQQQ | QQQQQQQQQQ | QQQQQQQQQQ | QQQQQPQTPG |
| 790 | 800 | 810 | 820 | 830 | 840 |
| SSTPKNEVHK | STQALHNTNL | TKEVRPLSAS | QPSLPHEVST | LISRPHPTVG | ESLASIPQPV |
| 850 | 860 | 870 | 880 | 890 | 900 |
| AAVHSTGLQA | GSRSTVPQRV | TLFRQMSSGA | IPPNRGVPPA | PPPPAAVQRE | SPSVLNTDPD |
| AEKPRFASNL |