O58663
Gene name |
PH0952 |
Protein name |
HTH arsR-type domain-containing protein |
Names |
|
Species |
Pyrococcus horikoshii (strain ATCC 700860 / DSM 12428 / JCM 9974 / NBRC 100139 / OT-3) |
KEGG Pathway |
pho:PH0952 |
EC number |
|
Protein Class |
LEUCINE-RICH REPEAT-CONTAINING PROTEIN (PTHR11017) |
Descriptions
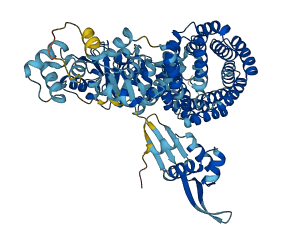
Signal transduction ATPase with numerous domains (STANDs), initially in monomeric resting forms, multimerize into large hubs that activate target macromolecules. Autoinhibitory interactions maintain the STAND conserved core (the NOD) closed in the absence of an inducer. In resting STAND proteins with a TPR sensor domain, the sensor domain establishes interactions with the NOD that strengthens the arm-based autoinhibition of STAND proteins. The interactions are disrupted in the multimerization-competent forms upon addition of its inducer. Similar interactions exist in a STAND with TPR sensor domain, PH0952 from Pyrococcus horikoshii, contributing to its autoinhibition.
Autoinhibitory domains (AIDs)
Target domain |
102-395 (NOD module with three domains including nucleotide-binding domain, the helical domain, and the winged-helix domain) |
Relief mechanism |
Ligand binding |
Assay |
Structural analysis |
Target domain |
102-395 (NOD module with three domains including nucleotide-binding domain, the helical domain, and the winged-helix domain) |
Relief mechanism |
Ligand binding |
Assay |
Structural analysis |
Accessory elements
No accessory elements
References
Autoinhibited structure
Activated structure

2 structures for O58663
| Entry ID | Method | Resolution | Chain | Position | Source |
|---|---|---|---|---|---|
| 6MFV | X-ray | 340 A | A/B/C/D | 102-753 | PDB |
| AF-O58663-F1 | Predicted | AlphaFoldDB |
No variants for O58663
| Variant ID(s) | Position | Change | Description | Diseaes Association | Provenance |
|---|---|---|---|---|---|
| No variants for O58663 | |||||
No associated diseases with O58663
No regional properties for O58663
| Type | Name | Position | InterPro Accession |
|---|---|---|---|
| No domain, repeats, and functional sites for O58663 | |||
Functions
| Description | ||
|---|---|---|
| EC Number | ||
| Subcellular Localization |
|
|
| PANTHER Family | PTHR11017 | LEUCINE-RICH REPEAT-CONTAINING PROTEIN |
| PANTHER Subfamily | PTHR11017:SF597 | TIR DOMAIN-CONTAINING PROTEIN |
| PANTHER Protein Class | ||
| PANTHER Pathway Category | No pathway information available | |
No GO annotations of cellular component
| Name | Definition |
|---|---|
| No GO annotations for cellular component |
2 GO annotations of molecular function
| Name | Definition |
|---|---|
| ADP binding | Binding to ADP, adenosine 5'-diphosphate. |
| DNA-binding transcription factor activity | A transcription regulator activity that modulates transcription of gene sets via selective and non-covalent binding to a specific double-stranded genomic DNA sequence (sometimes referred to as a motif) within a cis-regulatory region. Regulatory regions include promoters (proximal and distal) and enhancers. Genes are transcriptional units, and include bacterial operons. |
No GO annotations of biological process
| Name | Definition |
|---|---|
| No GO annotations for biological process |
No homologous proteins in AiPD
| UniProt AC | Gene Name | Protein Name | Species | Evidence Code |
|---|---|---|---|---|
| No homologous proteins | ||||
| 10 | 20 | 30 | 40 | 50 | 60 |
| MCMLEPSKIF | KALSNPINLK | ILTLLRSSSF | HPRELARILN | RDETDISRRL | RQLERLGLIK |
| 70 | 80 | 90 | 100 | 110 | 120 |
| GKWERVDGKN | VRVYSLKVSE | IRIFMHPTKL | EVKVGENESY | EAPIEWESSP | RVEVFVGRKR |
| 130 | 140 | 150 | 160 | 170 | 180 |
| ELSIIRNAKG | VVVIYGIAGI | GKTSLAAKAF | PNAYWYNVTG | LEDFKYFAWQ | LGLFLSSIGF |
| 190 | 200 | 210 | 220 | 230 | 240 |
| EDLLEYLRGG | GNNENDIFKL | ITEGIEKTGA | IIIIDDFHKF | QDEKVNYLLS | YLAPRIKKGK |
| 250 | 260 | 270 | 280 | 290 | 300 |
| VIITTRIRPN | LGNEGVTYVN | LKGLNPEEAY | SLAREKEKSM | TPEEFAKLYK | LTFGHPLMLN |
| 310 | 320 | 330 | 340 | 350 | 360 |
| LILESSEILA | TGKDTVFNFL | FEEVYQMLNE | EEKDLLSILS | LFDEPIEYEG | IKFLYDRNPF |
| 370 | 380 | 390 | 400 | 410 | 420 |
| VPLYSLMKKG | LIEKKGEKYF | VHDMVREFVR | EVSNQEEKEV | YLRHVNFLLK | SKTPINFLRA |
| 430 | 440 | 450 | 460 | 470 | 480 |
| FKYAIKVGSS | ELIRNLVELR | VKEFYRIIVD | FPRMYQRLLM | EVEDNPYAKI | EIAIIEVQRG |
| 490 | 500 | 510 | 520 | 530 | 540 |
| LFEKAIKLLK | EAEPYVDEFF | KCEIYSWLAD | AYMELENLEK | AERYLKKTKE | IVEKINDMYA |
| 550 | 560 | 570 | 580 | 590 | 600 |
| WFSYYAEKTK | YEYYKENSRE | ALKSALKELE | IIRKIGDPEK | EGLVLLHVGD | IYLHMGNYEK |
| 610 | 620 | 630 | 640 | 650 | 660 |
| GISYYQEALK | MAKAYGIKFL | EHISYMELAK | GYYQLKLYEK | ASEYSEKAAN | YFLMIRNYRR |
| 670 | 680 | 690 | 700 | 710 | 720 |
| ATDAMAYGSV | SYIATKNLEK | AEKFAKEMIR | IAQSTDYPLA | WAGYIFLAAV | DFLKGDDWRE |
| 730 | 740 | 750 | |||
| DYNLGKAHLK | EYPWLFEAVL | DELKKVFDLS | NFK |