O55033
Gene name |
Nck2 (Grb4) |
Protein name |
Cytoplasmic protein NCK2 |
Names |
Growth factor receptor-bound protein 4, NCK adaptor protein 2, Nck-2, SH2/SH3 adaptor protein NCK-beta |
Species |
Mus musculus (Mouse) |
KEGG Pathway |
mmu:17974 |
EC number |
|
Protein Class |
|
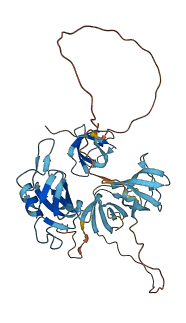
Descriptions
Autoinhibitory domains (AIDs)
Target domain |
111-170 (SH3.2 domain) |
Relief mechanism |
PTM |
Assay |
|
Accessory elements
No accessory elements
References
- Takeuchi K et al. (2010) "Autoinhibitory interaction in the multidomain adaptor protein Nck: possible roles in improving specificity and functional diversity", Biochemistry, 49, 5634-41
- Ogawa S et al. (1994) "The C-terminal SH3 domain of the mouse c-Crk protein negatively regulates tyrosine-phosphorylation of Crk associated p130 in rat 3Y1 cells", Oncogene, 9, 1669-78
- Sriram G et al. (2012) "Commentary: The carboxyl-terminal Crk SH3 domain: Regulatory strategies and new perspectives", FEBS letters, 586, 2615-8
- Kobashigawa Y et al. (2007) "Structural basis for the transforming activity of human cancer-related signaling adaptor protein CRK", Nature structural & molecular biology, 14, 503-10
Autoinhibited structure

Activated structure

1 structures for O55033
| Entry ID | Method | Resolution | Chain | Position | Source |
|---|---|---|---|---|---|
| AF-O55033-F1 | Predicted | AlphaFoldDB |
2 variants for O55033
| Variant ID(s) | Position | Change | Description | Diseaes Association | Provenance |
|---|---|---|---|---|---|
| rs216662714 | 123 | V>A | No | Ensembl | |
| rs864287274 | 242 | G>D | No | Ensembl |
No associated diseases with O55033
8 regional properties for O55033
| Type | Name | Position | InterPro Accession |
|---|---|---|---|
| domain | SH2 domain | 283 - 379 | IPR000980 |
| domain | SH3 domain | 2 - 61 | IPR001452-1 |
| domain | SH3 domain | 111 - 170 | IPR001452-2 |
| domain | SH3 domain | 195 - 257 | IPR001452-3 |
| domain | Nck2, SH3 domain 1 | 2 - 59 | IPR035559 |
| domain | Nck2, SH3 domain 2 | 114 - 168 | IPR035560 |
| domain | Nck2, SH3 domain 3 | 198 - 256 | IPR035561 |
| domain | Nck2, SH2 domain | 283 - 380 | IPR035883 |
6 GO annotations of cellular component
| Name | Definition |
|---|---|
| cytoplasm | The contents of a cell excluding the plasma membrane and nucleus, but including other subcellular structures. |
| cytosol | The part of the cytoplasm that does not contain organelles but which does contain other particulate matter, such as protein complexes. |
| endoplasmic reticulum | The irregular network of unit membranes, visible only by electron microscopy, that occurs in the cytoplasm of many eukaryotic cells. The membranes form a complex meshwork of tubular channels, which are often expanded into slitlike cavities called cisternae. The ER takes two forms, rough (or granular), with ribosomes adhering to the outer surface, and smooth (with no ribosomes attached). |
| postsynaptic density | An electron dense network of proteins within and adjacent to the postsynaptic membrane of an asymmetric, neuron-neuron synapse. Its major components include neurotransmitter receptors and the proteins that spatially and functionally organize them such as anchoring and scaffolding molecules, signaling enzymes and cytoskeletal components. |
| synapse | The junction between an axon of one neuron and a dendrite of another neuron, a muscle fiber or a glial cell. As the axon approaches the synapse it enlarges into a specialized structure, the presynaptic terminal bouton, which contains mitochondria and synaptic vesicles. At the tip of the terminal bouton is the presynaptic membrane; facing it, and separated from it by a minute cleft (the synaptic cleft) is a specialized area of membrane on the receiving cell, known as the postsynaptic membrane. In response to the arrival of nerve impulses, the presynaptic terminal bouton secretes molecules of neurotransmitters into the synaptic cleft. These diffuse across the cleft and transmit the signal to the postsynaptic membrane. |
| vesicle membrane | The lipid bilayer surrounding any membrane-bounded vesicle in the cell. |
5 GO annotations of molecular function
| Name | Definition |
|---|---|
| phosphotyrosine residue binding | Binding to a phosphorylated tyrosine residue within a protein. |
| protein-containing complex binding | Binding to a macromolecular complex. |
| receptor tyrosine kinase binding | Binding to a receptor that possesses protein tyrosine kinase activity. |
| scaffold protein binding | Binding to a scaffold protein. Scaffold proteins are crucial regulators of many key signaling pathways. Although not strictly defined in function, they are known to interact and/or bind with multiple members of a signaling pathway, tethering them into complexes. |
| signaling adaptor activity | The binding activity of a molecule that brings together two or more molecules in a signaling pathway, permitting those molecules to function in a coordinated way. Adaptor molecules themselves do not have catalytic activity. |
16 GO annotations of biological process
| Name | Definition |
|---|---|
| actin filament organization | A process that is carried out at the cellular level which results in the assembly, arrangement of constituent parts, or disassembly of cytoskeletal structures comprising actin filaments. Includes processes that control the spatial distribution of actin filaments, such as organizing filaments into meshworks, bundles, or other structures, as by cross-linking. |
| cell migration | The controlled self-propelled movement of a cell from one site to a destination guided by molecular cues. Cell migration is a central process in the development and maintenance of multicellular organisms. |
| dendritic spine development | The process whose specific outcome is the progression of the dendritic spine over time, from its formation to the mature structure. A dendritic spine is a protrusion from a dendrite and a specialized subcellular compartment involved in synaptic transmission. |
| ephrin receptor signaling pathway | The series of molecular signals initiated by ephrin binding to its receptor, and ending with the regulation of a downstream cellular process, e.g. transcription. |
| immunological synapse formation | The formation of an area of close contact between a lymphocyte (T-, B-, or natural killer cell) and a target cell through the clustering of particular signaling and adhesion molecules and their associated membrane rafts on both the lymphocyte and target cell, which facilitates activation of the lymphocyte, transfer of membrane from the target cell to the lymphocyte, and in some situations killing of the target cell through release of secretory granules and/or death-pathway ligand-receptor interaction. |
| lamellipodium assembly | Formation of a lamellipodium, a thin sheetlike extension of the surface of a migrating cell. |
| negative regulation of endoplasmic reticulum stress-induced eIF2 alpha phosphorylation | Any process that stops, prevents or reduces the frequency, rate or extent of endoplasmic reticulum stress-induced eiF2alpha phosphorylation. |
| negative regulation of peptidyl-serine phosphorylation | Any process that stops, prevents, or reduces the frequency, rate or extent of the phosphorylation of peptidyl-serine. |
| negative regulation of PERK-mediated unfolded protein response | Any process that stops, prevents or reduces the frequency, rate or extent of the PERK-mediated unfolded protein response. |
| negative regulation of transcription from RNA polymerase II promoter in response to endoplasmic reticulum stress | Any process that stops, prevents, or reduces the frequency, rate or extent of transcription from an RNA polymerase II promoter as a result of an endoplasmic reticulum stress. |
| positive regulation of actin filament polymerization | Any process that activates or increases the frequency, rate or extent of actin polymerization. |
| positive regulation of endoplasmic reticulum stress-induced intrinsic apoptotic signaling pathway | Any process that activates or increases the frequency, rate or extent of an endoplasmic reticulum stress-induced intrinsic apoptotic signaling pathway. |
| positive regulation of T cell proliferation | Any process that activates or increases the rate or extent of T cell proliferation. |
| positive regulation of transcription by RNA polymerase II | Any process that activates or increases the frequency, rate or extent of transcription from an RNA polymerase II promoter. |
| positive regulation of translation in response to endoplasmic reticulum stress | Any process that activates, or increases the frequency, rate or extent of translation as a result of endoplasmic reticulum stress. |
| signal transduction | The cellular process in which a signal is conveyed to trigger a change in the activity or state of a cell. Signal transduction begins with reception of a signal (e.g. a ligand binding to a receptor or receptor activation by a stimulus such as light), or for signal transduction in the absence of ligand, signal-withdrawal or the activity of a constitutively active receptor. Signal transduction ends with regulation of a downstream cellular process, e.g. regulation of transcription or regulation of a metabolic process. Signal transduction covers signaling from receptors located on the surface of the cell and signaling via molecules located within the cell. For signaling between cells, signal transduction is restricted to events at and within the receiving cell. |
5 homologous proteins in AiPD
| UniProt AC | Gene Name | Protein Name | Species | Evidence Code |
|---|---|---|---|---|
| P16333 | NCK1 | Cytoplasmic protein NCK1 | Homo sapiens (Human) | PR |
| O43639 | NCK2 | Cytoplasmic protein NCK2 | Homo sapiens (Human) | EV |
| Q99M51 | Nck1 | Cytoplasmic protein NCK1 | Mus musculus (Mouse) | PR |
| Q64010 | Crk | Adapter molecule crk | Mus musculus (Mouse) | EV SS |
| P47941 | Crkl | Crk-like protein | Mus musculus (Mouse) | SS |
| 10 | 20 | 30 | 40 | 50 | 60 |
| MTEEVIVIAK | WDYTAQQDQE | LDIRKNERLW | LLDDSKTWWR | VRNAANRTGY | VPSNYVERKN |
| 70 | 80 | 90 | 100 | 110 | 120 |
| SLKKGSLVKN | LKDTLGLGKT | RRKPSARDAS | PTPSTDAEYP | ANGSGADRIY | DLNIPAFVKF |
| 130 | 140 | 150 | 160 | 170 | 180 |
| AYVAEREDEL | SLVKGSRVTV | MEKCSDGWWR | GSFNGQIGWF | PSNYVLEEAD | EAAAEAPSFL |
| 190 | 200 | 210 | 220 | 230 | 240 |
| SLRRGTALSN | GQGARVLHVV | QTLYPFSSVT | EEELSFEKGE | TMEVIEKPEN | DPEWWKCKNA |
| 250 | 260 | 270 | 280 | 290 | 300 |
| RGQVGLVPKN | YVVVLSDGPA | LHPAHTPQIS | YTGPSASGRF | AGREWYYGNV | TRHQAECALN |
| 310 | 320 | 330 | 340 | 350 | 360 |
| ERGVEGDFLI | RDSESSPSDF | SVSLKASGRN | KHFKVQLVDS | VYCIGQRRFH | SMDELVEHYK |
| 370 | |||||
| KAPIFTSEHG | EKLYLVRALQ |