O19132
Gene name |
NOS1 |
Protein name |
Nitric oxide synthase, brain |
Names |
Constitutive NOS, NC-NOS, NOS type I, Neuronal NOS, N-NOS, nNOS, Peptidyl-cysteine S-nitrosylase NOS1, bNOS |
Species |
Oryctolagus cuniculus (Rabbit) |
KEGG Pathway |
ocu:100009243 |
EC number |
1.14.13.39: With NADH or NADPH as one donor, and incorporation of one atom of oxygen |
Protein Class |
|
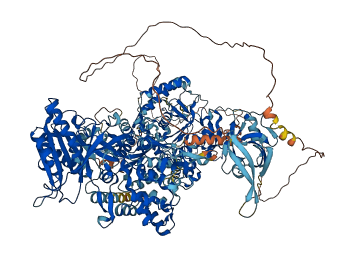
Descriptions
Autoinhibitory domains (AIDs)
Target domain |
357-718 (NO synthase domain); 996-1364 (FAD/NAD-binding domain) |
Relief mechanism |
Partner binding |
Assay |
|
Accessory elements
No accessory elements
Autoinhibited structure

Activated structure

1 structures for O19132
| Entry ID | Method | Resolution | Chain | Position | Source |
|---|---|---|---|---|---|
| AF-O19132-F1 | Predicted | AlphaFoldDB |
No variants for O19132
| Variant ID(s) | Position | Change | Description | Diseaes Association | Provenance |
|---|---|---|---|---|---|
| No variants for O19132 | |||||
No associated diseases with O19132
18 regional properties for O19132
| Type | Name | Position | InterPro Accession |
|---|---|---|---|
| domain | Flavodoxin-like | 762 - 775 | IPR001094-1 |
| domain | Flavodoxin-like | 809 - 820 | IPR001094-2 |
| domain | Flavodoxin-like | 882 - 892 | IPR001094-3 |
| domain | Flavodoxin-like | 906 - 925 | IPR001094-4 |
| domain | Oxidoreductase FAD/NAD(P)-binding | 1252 - 1364 | IPR001433 |
| domain | PDZ domain | 17 - 100 | IPR001478 |
| domain | Flavoprotein pyridine nucleotide cytochrome reductase | 1033 - 1043 | IPR001709-1 |
| domain | Flavoprotein pyridine nucleotide cytochrome reductase | 1179 - 1186 | IPR001709-2 |
| domain | Flavoprotein pyridine nucleotide cytochrome reductase | 1215 - 1224 | IPR001709-3 |
| domain | Flavoprotein pyridine nucleotide cytochrome reductase | 1251 - 1270 | IPR001709-4 |
| domain | Flavoprotein pyridine nucleotide cytochrome reductase | 1281 - 1290 | IPR001709-5 |
| domain | Flavoprotein pyridine nucleotide cytochrome reductase | 1294 - 1305 | IPR001709-6 |
| domain | Flavoprotein pyridine nucleotide cytochrome reductase | 1326 - 1342 | IPR001709-7 |
| domain | Flavoprotein pyridine nucleotide cytochrome reductase | 1351 - 1359 | IPR001709-8 |
| domain | Sulfite reductase [NADPH] flavoprotein alpha-component-like, FAD-binding | 992 - 1220 | IPR003097 |
| domain | Nitric oxide synthase, N-terminal | 357 - 718 | IPR004030 |
| domain | Flavodoxin/nitric oxide synthase | 761 - 941 | IPR008254 |
| domain | FAD-binding domain, ferredoxin reductase-type | 996 - 1243 | IPR017927 |
Functions
| Description | ||
|---|---|---|
| EC Number | 1.14.13.39 | With NADH or NADPH as one donor, and incorporation of one atom of oxygen |
| Subcellular Localization |
|
|
| PANTHER Family | ||
| PANTHER Subfamily | ||
| PANTHER Protein Class | ||
| PANTHER Pathway Category | No pathway information available | |
2 GO annotations of cellular component
| Name | Definition |
|---|---|
| dendritic spine | A small, membranous protrusion from a dendrite that forms a postsynaptic compartment, typically receiving input from a single presynapse. They function as partially isolated biochemical and an electrical compartments. Spine morphology is variable:they can be thin, stubby, mushroom, or branched, with a continuum of intermediate morphologies. They typically terminate in a bulb shape, linked to the dendritic shaft by a restriction. Spine remodeling is though to be involved in synaptic plasticity. |
| sarcolemma | The outer membrane of a muscle cell, consisting of the plasma membrane, a covering basement membrane (about 100 nm thick and sometimes common to more than one fiber), and the associated loose network of collagen fibers. |
7 GO annotations of molecular function
| Name | Definition |
|---|---|
| calmodulin binding | Binding to calmodulin, a calcium-binding protein with many roles, both in the calcium-bound and calcium-free states. |
| flavin adenine dinucleotide binding | Binding to FAD, flavin-adenine dinucleotide, the coenzyme or the prosthetic group of various flavoprotein oxidoreductase enzymes, in either the oxidized form, FAD, or the reduced form, FADH2. |
| FMN binding | Binding to flavin mono nucleotide. Flavin mono nucleotide (FMN) is the coenzyme or the prosthetic group of various flavoprotein oxidoreductase enzymes. |
| heme binding | Binding to a heme, a compound composed of iron complexed in a porphyrin (tetrapyrrole) ring. |
| metal ion binding | Binding to a metal ion. |
| NADP binding | Binding to nicotinamide-adenine dinucleotide phosphate, a coenzyme involved in many redox and biosynthetic reactions; binding may be to either the oxidized form, NADP+, or the reduced form, NADPH. |
| nitric-oxide synthase activity | Catalysis of the reaction: L-arginine + n NADPH + n H+ + m O2 = citrulline + nitric oxide + n NADP+. |
1 GO annotations of biological process
| Name | Definition |
|---|---|
| nitric oxide biosynthetic process | The chemical reactions and pathways resulting in the formation of nitric oxide, nitrogen monoxide (NO), a colorless gas only slightly soluble in water. |
No homologous proteins in AiPD
| UniProt AC | Gene Name | Protein Name | Species | Evidence Code |
|---|---|---|---|---|
| No homologous proteins | ||||
| 10 | 20 | 30 | 40 | 50 | 60 |
| MEEHVFGVQQ | IQPNVISVRL | FKRKVGGLGF | LVKERVSKPP | VIISDLIRGG | AAEQSGLIQA |
| 70 | 80 | 90 | 100 | 110 | 120 |
| GDIILAVNGR | PLVDLSYDSA | LEVLRGVASE | THVVLILRGP | EGFTTNLETT | FTGDGTPKTI |
| 130 | 140 | 150 | 160 | 170 | 180 |
| RVTQPLGAPT | KAVDLSHQPP | SAGKEQPRPV | DGAAGPGSWP | QPTQGHGQEA | GSPSRANGLA |
| 190 | 200 | 210 | 220 | 230 | 240 |
| PRTSSQDPAK | KSGWAGLQGS | GDKNELLKEI | EPVLTLLAGG | SKAVDGGGPA | KAETRDTGVQ |
| 250 | 260 | 270 | 280 | 290 | 300 |
| VDRDFDAKSH | KPLPLGVEND | RVFSDLWGKG | SAPVVLNNPY | SEKEQPPASG | KQSPTKNGSP |
| 310 | 320 | 330 | 340 | 350 | 360 |
| SKCPRFLKVK | NWETDVVLTD | TLHLKSTLET | GCTEHICMGS | IMFPSQHTRR | PEDIRTKEQL |
| 370 | 380 | 390 | 400 | 410 | 420 |
| FPLAKEFIDQ | YYSSIKRFGS | KAHMERLEEV | NKEIESTSTY | QLKDTELIYG | AKHAWRNASR |
| 430 | 440 | 450 | 460 | 470 | 480 |
| CVGRIQWSKL | QVFDARDCTT | AHGMFNYICN | HIKYATNKGN | LRSAITIFPQ | RTDGKHDFRV |
| 490 | 500 | 510 | 520 | 530 | 540 |
| WNSQLIRYAG | YKQPDGSTLG | DPANVQFTEI | CIQQGWKPPR | SRFDVLPLLL | QANGNDPELF |
| 550 | 560 | 570 | 580 | 590 | 600 |
| QIPPELVLEV | PIRHPKFEWF | KDLGLKWYGL | PAVSNMLLEI | GGLEFSACPF | SGWYMGTEIG |
| 610 | 620 | 630 | 640 | 650 | 660 |
| VRDYCDNSRY | NILEEVAKKM | NLDMRKTSSL | WKDQALVEIN | IAVLYSFQSD | KVTIVDHHSA |
| 670 | 680 | 690 | 700 | 710 | 720 |
| TESFIKHMEN | EYRCRGGCPA | DWVWIVPPMS | GSITPVFHQE | MLNYRLTPCF | EYQPDPWNTH |
| 730 | 740 | 750 | 760 | 770 | 780 |
| VWKGTNGTPT | KRRAIGFKKL | AEAVKFSAKL | MGQAMAKRVK | ATILYATETG | KSQAYAKTLC |
| 790 | 800 | 810 | 820 | 830 | 840 |
| EIFKHAFDAK | VMSMEEYDIV | HLEHETLVLV | VTSTFGNGDP | PENGEKFRCA | LMEMRHPNSL |
| 850 | 860 | 870 | 880 | 890 | 900 |
| QEERKSYKVR | FNSVSSYSDS | RKSSGDGPDV | RDHFESAGPL | ANVRFSVFGL | GSRAYPHFCA |
| 910 | 920 | 930 | 940 | 950 | 960 |
| FGHAVDTLLE | ELGGERILKM | REGDELCGQE | EAFRTWAKKV | FKAACDVFCV | GDDVNIEKAN |
| 970 | 980 | 990 | 1000 | 1010 | 1020 |
| NSLISNDRSW | KRNKFRLTYV | AEAPGLTQGL | SSVHKKRVSA | ARLLSRQNLQ | SPKSSRSTIF |
| 1030 | 1040 | 1050 | 1060 | 1070 | 1080 |
| VRLHTNGSQE | LQYQPGDHLG | VFPGNHEDLV | NALIERLEDA | PPANQMVKVE | LLEERNTALG |
| 1090 | 1100 | 1110 | 1120 | 1130 | 1140 |
| VISNWKDEPR | LPPCTVFQAF | KYYLDITTPP | TPLQLQQFAS | LASNEKEKQR | LLVLSKGLQE |
| 1150 | 1160 | 1170 | 1180 | 1190 | 1200 |
| YEEWKWGKNP | TIVEVLEEFP | SIQMPATLLL | TQLSLLQPRY | YSISSSPDMY | PDEVHLTVAI |
| 1210 | 1220 | 1230 | 1240 | 1250 | 1260 |
| VSYHTRDGEG | PIHHGVCSSW | LNRIPADEVV | PCFVRGAPSF | RLPRNPQVPC | ILVGPGTAFA |
| 1270 | 1280 | 1290 | 1300 | 1310 | 1320 |
| PFRSFWQQRQ | FDIQHKGMSP | CPMVLVFGCR | QSKIDHIYRE | EALQAKNKGV | FRELYTAYSR |
| 1330 | 1340 | 1350 | 1360 | 1370 | 1380 |
| EPDKPKKYVQ | DILQEQLAEQ | VYRALKEQGG | HIYVCGDVTM | AADVLKAVQR | IMAQQGKLSA |
| 1390 | 1400 | 1410 | 1420 | 1430 | |
| EDAGVFISRL | RDDNRYHEDI | FGVTLRTYEV | TNRLRSESIA | FIEESKKDTD | EVFSS |