F1Q4S1
Gene name |
atp9b |
Protein name |
Probable phospholipid-transporting ATPase IIB |
Names |
ATPase class II type 9B |
Species |
Danio rerio (Zebrafish) (Brachydanio rerio) |
KEGG Pathway |
|
EC number |
7.6.2.1: Linked to the hydrolysis of a nucleoside triphosphate |
Protein Class |
|
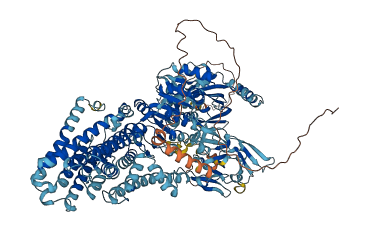
Descriptions
The autoinhibited protein was predicted that may have potential autoinhibitory elements via cis-regPred.
Autoinhibitory domains (AIDs)
Target domain |
|
Relief mechanism |
|
Assay |
cis-regPred |
Accessory elements
No accessory elements
Autoinhibited structure

Activated structure

1 structures for F1Q4S1
| Entry ID | Method | Resolution | Chain | Position | Source |
|---|---|---|---|---|---|
| AF-F1Q4S1-F1 | Predicted | AlphaFoldDB |
No variants for F1Q4S1
| Variant ID(s) | Position | Change | Description | Diseaes Association | Provenance |
|---|---|---|---|---|---|
| No variants for F1Q4S1 | |||||
No associated diseases with F1Q4S1
4 regional properties for F1Q4S1
Functions
| Description | ||
|---|---|---|
| EC Number | 7.6.2.1 | Linked to the hydrolysis of a nucleoside triphosphate |
| Subcellular Localization |
|
|
| PANTHER Family | ||
| PANTHER Subfamily | ||
| PANTHER Protein Class | ||
| PANTHER Pathway Category | No pathway information available | |
4 GO annotations of cellular component
| Name | Definition |
|---|---|
| endosome | A vacuole to which materials ingested by endocytosis are delivered. |
| integral component of membrane | The component of a membrane consisting of the gene products and protein complexes having at least some part of their peptide sequence embedded in the hydrophobic region of the membrane. |
| plasma membrane | The membrane surrounding a cell that separates the cell from its external environment. It consists of a phospholipid bilayer and associated proteins. |
| trans-Golgi network | The network of interconnected tubular and cisternal structures located within the Golgi apparatus on the side distal to the endoplasmic reticulum, from which secretory vesicles emerge. The trans-Golgi network is important in the later stages of protein secretion where it is thought to play a key role in the sorting and targeting of secreted proteins to the correct destination. |
4 GO annotations of molecular function
| Name | Definition |
|---|---|
| ATP binding | Binding to ATP, adenosine 5'-triphosphate, a universally important coenzyme and enzyme regulator. |
| ATP hydrolysis activity | Catalysis of the reaction: ATP + H2O = ADP + H+ phosphate. ATP hydrolysis is used in some reactions as an energy source, for example to catalyze a reaction or drive transport against a concentration gradient. |
| ATPase-coupled intramembrane lipid transporter activity | Catalysis of the movement of lipids from one membrane leaflet to the other, driven by ATP hydrolysis. This includes flippases and floppases. |
| magnesium ion binding | Binding to a magnesium (Mg) ion. |
3 GO annotations of biological process
| Name | Definition |
|---|---|
| endocytosis | A vesicle-mediated transport process in which cells take up external materials or membrane constituents by the invagination of a small region of the plasma membrane to form a new membrane-bounded vesicle. |
| phospholipid translocation | The movement of a phospholipid molecule from one leaflet of a membrane bilayer to the opposite leaflet. |
| retrograde vesicle-mediated transport, Golgi to endoplasmic reticulum | The directed movement of substances from the Golgi back to the endoplasmic reticulum, mediated by vesicles bearing specific protein coats such as COPI or COG. |
2 homologous proteins in AiPD
| 10 | 20 | 30 | 40 | 50 | 60 |
| MADGIPLNPV | RKNLRKTAYY | DASRPARYQI | EDESSNLDEM | PLMMSEEAFE | NDESDYQTLP |
| 70 | 80 | 90 | 100 | 110 | 120 |
| RARVSQRRRG | LGWFLCGGWK | VLCSSCCECL | VHTCRRKKEL | KARTVWLGHP | EKCEEKYPKN |
| 130 | 140 | 150 | 160 | 170 | 180 |
| AIKNQKYNIV | TFVPGVLYQQ | FKFFLNLYFL | VVACSQFVPS | LKIGYLYTYW | APLGFVLAVT |
| 190 | 200 | 210 | 220 | 230 | 240 |
| MVREAVDEVR | RCRRDKEMNS | QLYSKLTVRG | KVQVKSSDIQ | VGDLIIVEKN | QRIPADMIFL |
| 250 | 260 | 270 | 280 | 290 | 300 |
| RTSEKTGSCF | IRTDQLDGET | DWKLRIGVAC | TQRLPALGDL | FSISAYVYVQ | KPQLDIHSFE |
| 310 | 320 | 330 | 340 | 350 | 360 |
| GNFTREDCDP | PIHESLSIEN | TLWASTVVAS | GTVIGVVIYT | GKEMRSVMNT | SQSKNKVGLL |
| 370 | 380 | 390 | 400 | 410 | 420 |
| DLELNRLTKA | LFLAQVVLSV | VMVALQGFLG | PWFRNLFRFV | VLFSYIIPIS | LRVNLDMGKS |
| 430 | 440 | 450 | 460 | 470 | 480 |
| AYGWMIMKDE | NIPGTVVRTS | TIPEELGRLV | YLLTDKTGTL | TQNEMVFKRL | HLGTVSYGTD |
| 490 | 500 | 510 | 520 | 530 | 540 |
| TMDEIQSHII | QSYAQVSSAQ | SNGSSASSTP | SRKPQPPAPK | VRKSVSSRIH | EAVKAIALCH |
| 550 | 560 | 570 | 580 | 590 | 600 |
| NVTPVYESRV | NGANAEPEST | EADQDFSDDN | RTYQASSPDE | VALVRWTESV | GLTLVNRDLT |
| 610 | 620 | 630 | 640 | 650 | 660 |
| SLQLKTPAGQ | ILTYYILQIF | PFTSESKRMG | IIVREEATGD | ITFYMKGADV | AMASIVQYND |
| 670 | 680 | 690 | 700 | 710 | 720 |
| WLEEECGNMA | REGLRTLVVA | KKSLTEEQYQ | DFENRYNQAK | LSIHDRNLKV | AAVVESLERE |
| 730 | 740 | 750 | 760 | 770 | 780 |
| MELLCLTGVE | DQLQADVRPT | LELLRNAGIK | IWMLTGDKLE | TATCIAKSSH | LVSRNQDIHV |
| 790 | 800 | 810 | 820 | 830 | 840 |
| FKPVSNRGEA | HLELNAFRRK | HDCALVISGD | SLEVCLRYYE | HEFVELACQC | PAVVCCRCSP |
| 850 | 860 | 870 | 880 | 890 | 900 |
| TQKAQIVRLL | QQHTANRTCA | IGDGGNDVSM | IQAADCGIGI | EGKEGKQASL | AADFSITQFK |
| 910 | 920 | 930 | 940 | 950 | 960 |
| HIGRLLMVHG | RNSYKRSAAL | GQFVMHRGMI | ISTMQAVFSS | IFYFASVPLY | QGFLMVGYAT |
| 970 | 980 | 990 | 1000 | 1010 | 1020 |
| IYTMFPVFSL | VLDQDVKPEM | ALLYPELYKD | LTKGRSLSFK | TFLIWVLISI | YQGGILMYGA |
| 1030 | 1040 | 1050 | 1060 | 1070 | 1080 |
| LVLFDQEFVH | VVAISFTALI | LTELLMVALT | IRTWHWLMVV | AQLISLACYL | ASLAFLNEYF |
| 1090 | 1100 | 1110 | 1120 | ||
| DLSFITTRVF | LWKVCVITLV | SCLPLYIIKY | LKRKFSPPSY | SKLSS |